The effect of hyoscine butyl bromide on the active phase of labour among primiparous women delivered at the Jos University Teaching Hospital
Obikili Chinedu George FMCOG,FWACS1, Magaji Francis Ajang FMCOG,FWACS2, Anyaka Charles U FWACS2
Abstract
Background: Labour is a physiological process to expel products of conception to the external environment. More often than not, it results in the delivery of a healthy baby to a happy mother. However, it has also been associated with a significant number of adverse maternal and fetal outcomes. Over the years several steps and interventions have been taken to reduce these adverse outcomes. One of such intervention is the reduction of the duration of labour which has been found to improve neonatal outcomes. Hyoscine-Butyl Bromide has been shown to reduce the duration of labour, however, it is currently not recommended for labour intervention because no beneficial effects have been associated with its use yet and it remains an area prioritized for research.
Objective: To establish the effects of Hyoscine Butyl-bromide in the outcome of labour among primiparous women delivering at the Jos University Teaching Hospital (JUTH) and to contribute towards the management of these patients.
Design: This was a hospital-based double-blind placebo-controlled clinical trial
Methodology: In this study 120 women were recruited by convenience sampling and randomized into the 3 arms of the study. One arm received placebo, 2nd arm received 20mg of Hyoscine-Butyl Bromide and the 3rd arm received 40mg of Hyoscine-Butyl Bromide (HBB). Their labours were managed actively and outcomes were documented in a proforma. Statistical analysis was done using SPSS software version 20 and results calculated.
Results: Ninety-seven women had spontaneous vaginal delivery, 18 had Caesarean Sections and 5 had operative vaginal delivery. The mean duration of active phase of labour in the placebo arm was 325.84 minutes, it was 272.35 minutes in the 20mg HBB arm and 265.03 minutes in the 40mg HBB arm. This difference however was not shown to be statistically significant. There was no significant increase in adverse neonatal outcomes in any of the groups and there were no noted improved maternal or neonatal outcomes. Side effects were noted to be more in the 40mg HBB group.
Conclusion: There was an average of 53 minutes reduction in the duration of active phase of labour with the administration of 20mg of Hyoscine Buytl Bromide and a further average reduction of 7 minutes by an additional 20mg of Hyoscine Butyl Bromide.
There is not enough evidence from this study to recommend the use of hyoscine butyl bromide as an intervention in labour due to the absence of any beneficial effect and the presence of some unpleasant side effects.
Key Words; Hyoscine-Butyl Bromide, active phase, labour, Maternal Outcome, Primigravida, JUTH
Introduction
Labour is a physiological process during which the products of conception are expelled outside of the uterus. It is achieved with changes in the biochemical connective tissue and with gradual effacement and dilation of the uterine cervix1,2. This physiological process more often than not would go on uninterrupted resulting in the delivery of a healthy baby to a happy mother, however over the years various steps have been taken to help reduce the burden, pain, duration and complications that may occur as a result of this process, one of which is prolonged labour which has been linked with significant maternal and neonatal morbidity and mortality3.
The most notable step that has been taken to reduce prolonged labour is the active management of labour, which involves active phase interventions including artificial rupture of membranes, use of the partograph and augmentation of labour using oxytocin once contractions are determined to be inadequate4,5. These active phase interventions are important as it has been found that the duration of active phase of labour is an important determinant of maternal and fetal outcome6. However, these interventions have not solved all the issues of the labour process7 and there remain some issues with current interventions, for instance, amniotomy increases the risk for infection and oxytocin can cause hyperstimulation, water intoxication, vomiting, diarrhoea, fetal distress and neonatal jaundice8. Many works are still ongoing to ascertain if other interventions can aid in making the labour process shorter, more pleasant and with improved outcomes.
The use of antispasmodics for reducing the duration of labour was first described in 1937 by Hirsch, who reported a decrease in labour length by 2 – 4 hours following intrapartum administration of an antispasmodic-like drug, mainly among older nulliparous women8. Since then various works have been done to evaluate the use of antispasmodics in labour. A Cochrane review of 21 randomised controlled studies with a total of 3286 participants included, all types of antispasmodics were given at the beginning of established labour. They decreased the first stage of labour by 49 – 98 minutes as well as the total duration of labour by 49 to 121 minutes. The drugs did not affect the number of women requiring emergency caesarean sections and did not have serious side effects for either the mother or her baby. Since both maternal and neonatal adverse effects were poorly reported, it suggested that more information would be needed to make conclusions about the safety of these drugs during labour9.
In 2013, the World Health Organization (WHO) convened a Guideline Development Group (GDG) meeting on recommendations of augmentation of labour to assess evidence on effects on the pre-specified outcomes. The summary of the evidence showed that there was a significant reduction in the duration of the first stage of labour but no significant reduction in the duration of the second stage of labour10.
However, following the review, the WHO recommendation of 2014 was that “The use of antispasmodic agents for prevention of delay in labour is not recommended. (Weak recommendation, very low-quality evidence)”. This was because its beneficial effects were not fully established yet10.
Childbirth is a natural human activity that most women look forward to, however, the process of labour is often long and tumultuous leaving women in a state of disarray and distress. The use of antispasmodics in labour has long been considered as having possible beneficial effects.
However, details including its safety profile, appropriate dose, route of administration, improvement in maternal and fetal parameters are not fully answered. Also, as long as it is safe, affordable and feasible, most women would advocate for an intervention that can shorten their labour process.
Antispasmodics have been in the past demonstrated to reduce the total duration of labour however a lot of the previous works left some questions unanswered.
The GDG noted that the available data were too heterogeneous with respect to the participants and interventions to permit wide applicability of the results. The shortening in the length of the first stage of labour was considered inconsequential as it did not translate to improvement in other critical maternal or fetal outcomes. The GDG placed high value on safety issues, which were poorly reported and chose not to recommend the practice until new information demonstrating clinical benefits with minimal risks becomes available10.
The GDG considers the use of antispasmodic agents for treatment of delay in labour as a research priority and the WHO has placed the recommendation prioritized for updating in 201810. A recommended question asked by the GDG was “In pregnant women in labour, does use of antispasmodic agents for prevention of delay in labour, compared to no intervention improve maternal and perinatal outcomes?”10.
This study aims to use a homogenous population of women and a varying dosage of antispasmodic to possibly to access the effect of HBB on outcomes whilst putting into account the safety profile of the drugs and monitoring for any potential adverse effects.
The aim was to establish the effects of Hyoscine Butyl-bromide in the outcome of labour among women delivering at the Jos University Teaching Hospital (JUTH) and to contribute towards the management of these patients
The Objectives were to determine the effect of hyoscine butyl-bromide on the active phase of labour in primiparous women as compared to a control group.
To determine the effects of hyoscine butyl-bromide on the outcome and duration of active phase of labour
To determine if there is a dose dependent effect of hyoscine butyl-bromide in the active phase of labour
To determine the side-effect profile of hyoscine butyl-bromide in labour
To make recommendations on the use of hyoscine butyl-bromide in labour based on the findings in objectives ii, iii and iv above.
Null Hypothesis
Hyoscine butyl-bromide does not change the duration of active phase of labour in primiparous women in JUTH.
Materials and Methods
Study Area
The study was conducted in the Jos University Teaching Hospital (JUTH), a 600-bed tertiary health institution located in Jos, the capital of Plateau State in North Central Nigeria
Trial Design
This was a hospital-based double-blind placebo-controlled clinical trial
Sampling Technique
This was by consecutive sampling technique
Study Population
The study population comprised of one hundred and twenty women making up the placebo and HBB groups. All women were primiparous at onset of active phase of labour presenting to the labour ward of the Jos University Teaching Hospital. Forty were grouped into the placebo group, forty into the 20mg HBB group and forty into the 40mg HBB group.
Sample Size
A sample of 120 women were recruited for this study. Sample size was calculated using the formula for comparison of means with mean duration for control and HBB group gotten from a similar study conducted in Ile-Ife, Nigeria18.
Participants
Inclusion criteria
- Primiparous women at term with singleton pregnancy
- Presentation in spontaneous labour at cervical os dilation of 4cm or in latent phase of labour
- Primiparous women meeting the above criteria who gave consent to participate in the study
Exclusion criteria
- Women with any contraindication to vaginal delivery
- Women with history of previous adverse drug reaction to hyoscine butyl-bromide
- Women with any contraindication to hyoscine butyl-bromide (History of gastric reflux disease, ulcerative colitis, severe constipation, mechanical bowel obstruction, megacolon, paralytic ileus, hypertension, acute narrow angle glaucoma, palpitations, Downs syndrome, autonomic neuropathy, myasthenia gravis and allergic reaction to hyoscine butyl bromide)
- Women taking medications with drug interaction to hyoscine butyl-bromide (antihistamines, ipratropium, metoclopramide and tricyclic antidepressants)
Recruitment
Eligible primiparous pregnant women presenting to the labour ward in latent phase of labour or at onset of active phase of labour were recruited into the study population.
Randomisation
Sequence generation
The participants were recruited consecutively and randomisation done by balloting. The women were asked to pick unlabelled envelopes from a box.
Allocation concealment
Injections had been prepared, with A containing placebo (2mls of Normal saline), B containing 20mg of Hyoscine Butyl-Bromide (1ml of 20mg HBB and 1ml of Normal Saline) and C containing 40mg Hyoscine Butyl-Bromide (2mls of HBB)
All the injections were labelled A, B or C, contained 2mls of clear fluid and kept in the refrigerator.
Implementation
Each participant picked an envelop labeled A, B or C and the accoucheur collected the equivalent injection label from the refrigerator and administered the fluid intravenously at the onset of active phase of labour
A structured proforma was administered, and privacy ensured while interviews were being conducted. Serial numbers were assigned to each patient to protect her identity and eliminate bias.
Blinding
It was a double-blind clinical trial. The participants did not know what was the content of the injection, as they were all clear fluids of 2mls. The experimenters/doctors also did not know what was the content of the injection as a prepared injection with labeling was taken from the refrigerator and administered
The statistical analysis was done with the awareness of what each participant received
Interventions
At the onset of active phase of labour every woman was admitted into the labour room, a single dose of an injection labelled A, B or C was given intravenously (with A being 2ml of normal saline, B being 1ml of 20mg HBB + 1 ml normal saline and C being 2mls, equating to 40mg of HBB).
Protocol for management of labour
The labour process of each woman was managed using the active management of labour protocols. Onset of active phase of labour was diagnosed by an experienced doctor, with minimum level of resident doctor with at least one year of practice in the department of Obstetrics and Gynaecology at JUTH. Artificial rupture of membrane was done at this time and the progress of labour monitored used the partograph. Labour analgesia was given, either pentazocine or pethidine to every woman. There was labour ward companionship of one relative of the patient’s choice through out the duration of labour.
Uterine contractions were documented and augmentation of labour with 5 IU of oxytocin in 500mls of intravenous fluids was instituted when uterine contractions were not adequate. A minimum of 3 strong contractions lasting at least 45 seconds over a 10-minute period was considered as adequate. Oxytocin augmentation was started at 10 drops per minute and increased by 10 drops per minute every 30 minutes until contractions were adequate.
Outcomes
Any adverse side effect was documented in the proforma, with specific questions including sleepiness, vision changes, dry mouth, dry skin, difficulty in passing urine, dizziness, diarrhoea, rapid heart rate and severe allergic reaction. Any fetal heart rate abnormalities were also documented in the proforma.
Following delivery, for those who achieve vaginal delivery, the total duration of the active phase of labour was calculated and documented. Those who had caesarean sections were documented with reasons. The fetal outcomes were taken, with the APGAR scores and admissions to the SCBU with reasons documented.
The side effects and fetal wellbeing were enquired into, at least 12 hours after delivery and before discharge.
Since the study involved a single dose of injection, it was impossible to withdraw women from the study. Thus, all untoward effects were reported. However, there was no untoward side effect that would have necessitated withdrawal.
Data Analysis
All statistical analysis was performed using SPSS software (version 20).
A P-value of less or equal 0.05 was accepted as indicating statistical significance.
Ethical Consideration
Ethical clearance was obtained from the Ethical committee of the Jos University Teaching Hospital (JUTH).
Results
Recruitment
A total of one hundred and twenty women were recruited into this study. Recruitment commenced in July 2019 and was completed in April, 2020. The trial ended in April, 2020 following recruitment of desired sample size.
Numbers Analyzed
Forty participants were recruited into each group and each analyzed according to their originally assigned intervention.
Baseline Data
The sociodemographic characteristics of the women that participated in the study is shown in Table 1. The overall mean age was 25.42 +/- 4.39 with a median of 26 years and an age range of 15 to 37 years.
There were 3 arms of the study, the first arm received placebo, the second arm received 20mg of Hyoscine Butyl-Bromide and the third arm received 40mg of Hyoscine Butyl-Bromide. 40 participants were recruited into each of the groups.
Table 1: Sociodemographic characteristics of participants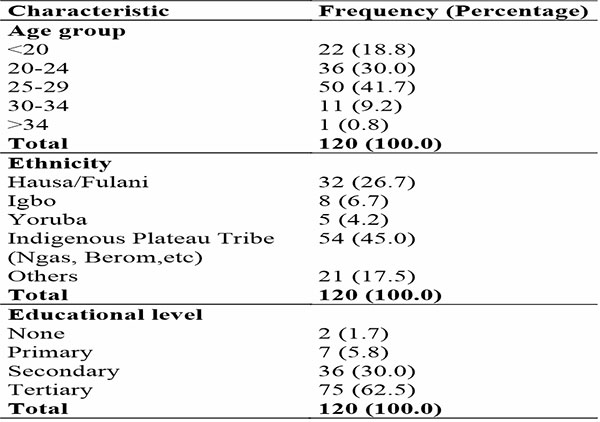
Table 2: Showing sociodemographic distribution of the different arms of the study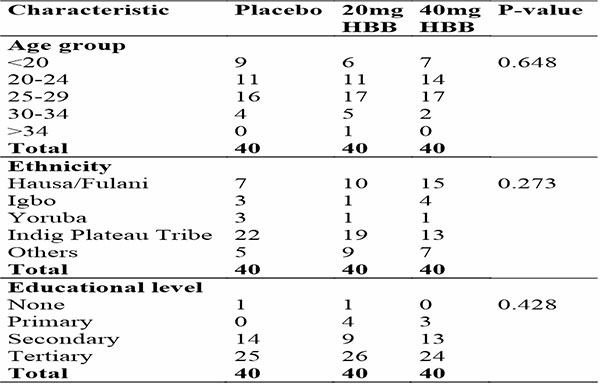
Table 3: Showing the outcome of labour
Table 4: Showing outcome of labour in the different arms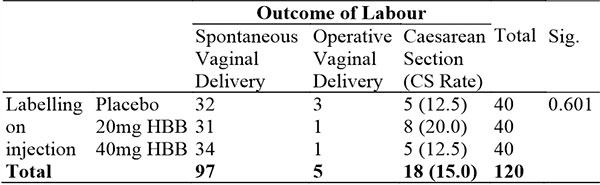
Table 5: Showing duration of active phase of labour in different arms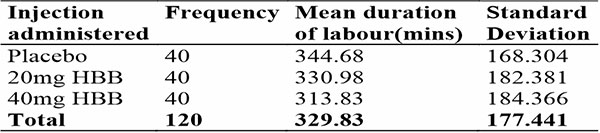
Table 6: Showing duration of active phase of labour in different arms amongst participants that had Spontaneous Vaginal Delivery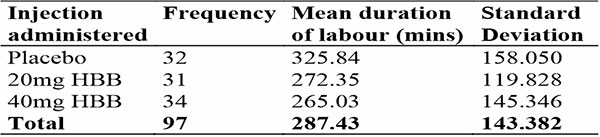
Table 7: Showing ANOVA relationship between injection administered and duration of active phase of labour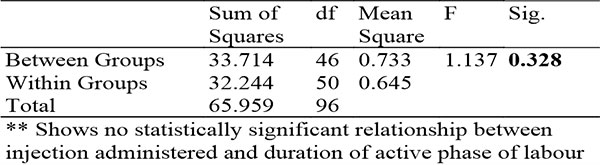
Table 8: Showing Pearson’s Correlation between injection administered and duration of active phase of labour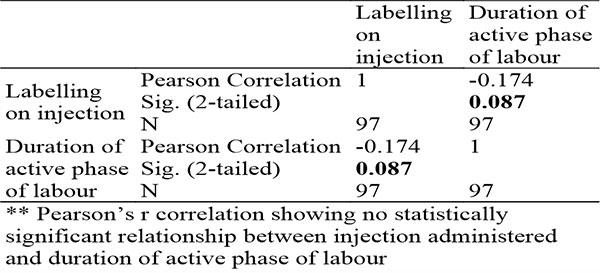
Table 9: Showing median 1st and 5th minute APGAR scores in different arms of the study
Table 10: Showing ANOVA for relationship between injection administered and 1st minute APGAR score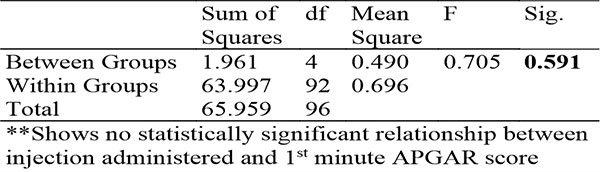
Table 11: Showing ANOVA for relationship between injection administered and 5th minute APGAR score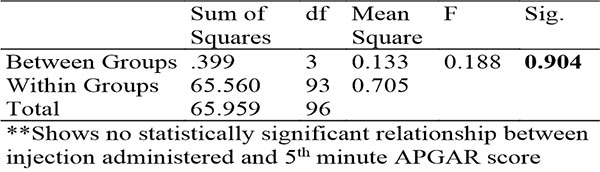
Table 12: Sowing crosstabulation between injection administered and FHR abnormalities during labour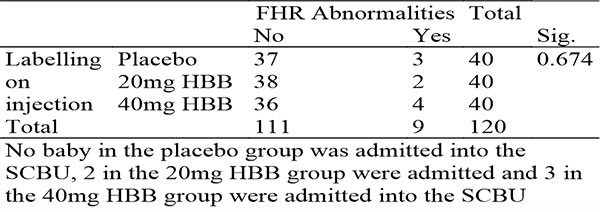
Table 13: Showing crosstabulation on injection administered and admission into the NICU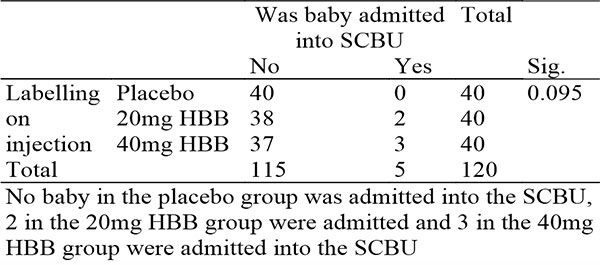
Table 14: Showing crosstabulation between injection administered and fetal well being 12 hours post delivery
Table 15: Showing crosstabulation between injection administered and maternal side effects during labour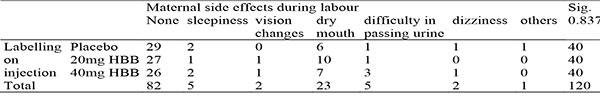
Table 16: Showing crosstabulation between injection administered and maternal side effects 12 hours post partum
Discussion
Prolonged labour is a significant source of maternal and neonatal morbidity and mortality particularly in the developing world3, several works have been done in an attempt to reduce the incidence of prolonged6.
In this study, 80.8% of participants had spontaneous vaginal delivery while the Caesarean Section rate was 18% with 4.2% having an instrumental vaginal delivery. This was similar to previous studies done in this region with a CS rate of 21.4% in Abuja27 reported and 19.6% in Jos28. The instrumental delivery rate was similar to findings from other Nigerian studies ranging from 0.67% to 28.7%29.
The caesarean section rates and operative vaginal delivery rates did not significantly differ in the different arms of the study or appear to show a trend by the dosage of HBB as seen in table 4. This was similar to previous studies which found no decrease in CS rates with the administration of HBB9.
The mean duration of active phase of labour was 329.83 minutes in total. In women who achieved spontaneous vaginal delivery, it was 287.43 minutes. This is within the expected range of 222 – 354 minutes for nulliparous women30.
There was a progressive reduction in the duration of active phase of labour with the placebo arm having the longest duration of labour and the 40mg HBB arm having the shortest duration of labour as seen in tables 5 and 6.
The duration of labour on average was shortened by 53 minutes in the 20mg HBB group and shorter by a further 7 minutes in the 40mg HBB group. These findings were similar to the conclusions from the Cochrane collaborative review which found a decrease in duration of labour by 49 to 121 minutes9. It was also similar to studies from South western Nigeria18. However, it is at variance with an Abuja study which suggested no difference in duration of labour with the drug arm having a slightly longer labour duration26. The Abuja study, however, used the Intra-muscular route, as opposed to the intra-venous route in their work.
Thus, this suggests the possibility that route of administration could be a factor in its effect and might need to be explored further.
The 2 drug arms of this study varied by just 7 minutes and it was found to not be statistically significant. The findings suggests that dose dependent effects of HBB are minimal.
There was no significant difference in the APGAR scores in the different arms of the study with a median of 8 for the first minute APGAR in all groups and a median of 9 for the fifth minute APGAR in all groups.
These findings suggest that there is no obvious harmful effect of HBB on the immediate neonatal period though the findings were not conclusive.
There was also no statistically significant difference in FHR abnormalities during labour in all 3 arms of the study, with an average of 3 noted FHR abnormalities per arm.
There was an increase in the incidence of admission into the NICU in the drug arm, which was higher in the 40mg group. With no admission in the placebo group, 2 admissions in the 20mg HBB group and 3 admissions in the 40mg HBB group. Two of the admissions were due to fetal macrosomia, which was unrelated to labour intervention, whilst the other 3 were not stated.
However, this needs to be explored further, as safety profile of HBB use in labour is a priority area and needs to be clearly established. There was no significant difference in the fetal well being 12 hours post-delivery, though the group which received 40mg HBB had 2 complaints, fetal hypoglycemia following birth from a woman with GDM and difficulty in breathing due to congenital pneumonia. Neither of which has been linked with HBB.
More work needs to be done. But from this study, there appears to be no significant adverse perinatal outcome from the use of HBB. This is in keeping with previous studies which showed no difference in APGAR scores and other fetal outcomes21,22,25.
The most common maternal side effect was dry mouth. This was highest in the 20mg arm of the study. Though, 6 cases were seen in the placebo group, as dry mouth is a common complaint in normal labour, as well as a noted side effect of HBB15. Other side effects included difficulty in passing urine which occurred 3 times in the 40mg arm of the study and once in the 20mg and placebo arms. Vision changes also occurred once in both 20mg and 40mg groups but none in the placebo arm. The other side effects such as sleepiness and dizziness did not vary significantly in the 3 arms of this study.
By 12 hours post-delivery 4 women in the 40mg HBB arm complained of dry mouth as opposed to none in either of the other two arms, with two women complaining of sleepiness whilst there was none in the placebo or 20mg arm as seen in Table 17.
Overall, the side effects of administration of HBB did not differ from what would be expected in the normal none pregnant population and there was no report of any severe adverse effect or reaction. Though, the 40mg arm of HBB had more and longer lasting side effects.
Conclusion
In conclusion, there was an average of 53 minutes reduction in the duration of active phase of labour with the administration of 20mg of Hyoscine Buytl Bromide and a further average reduction of 7 minutes by an additional 20mg of Hyoscine Butyl Bromide. There was no statistically significant difference in the maternal or neonatal outcomes between the three arms, thus, despite a slight decrease in duration of labour there was no demonstrable benefit to either the baby or the mother. Rather, there was a slight increase in maternal side effect, particularly in the 40mg arm of the study.
There is not enough evidence from this study to recommend the use of hyoscine butyl bromide as an intervention in labour due to the absence of any beneficial effect and the presence of some unpleasant side effects.
Limitations of the study
- The diagnosis of active phase of labour can be subjective and sometimes depends on the attending obstetrician, however, this remains the most accurate method and for this study experienced obstetricians and residents in training were used for uniformity as much as possible.
- The exact onset of active phase of labour can rarely be accurately determined, thus for this study the time of admission at 4cm and ARM was used as the time of onset.
- The onset of second stage of labour is also difficult to ascertain as four hourly reviews are conducted, thus the duration of active phase used will be a combination of active phase and second stage as it is expected to give more uniformity and a more objective analysis. That is from the onset of active phase till the time of delivery.
- Some of the side effects of HBB including sleepiness, vision changes, dry mouth, dry skin, difficulty in passing urine, dizziness and diarrhoea can also occur physiologically during labour, however their frequencies of occurrence in the different groups will help determine if HBB can be an attributable cause
- Follow-up of these patients for long-term effects was not done, a follow up study to ascertain the long-term safety profiles might be necessary.
- Statistical analysis was not blinded and this has the potential to create bias during the analysis.
Generalisability
The findings from this study can be generalized to the overall target population as the sociodemographic distribution is a fair representation of the overall population. Despite the fact that it used a population of primiparous women alone, the essence was to evaluate the effects of HBB, reducing confounders. Thus, multiparous women are also likely to have similar response to HBB.
Recommendations
- Hyoscine Butyl Bromide should not be used as a labour intervention for now until more works are done to clearly demonstrate its safety and potential benefits of its use
- Larger scale follow up studies should be done to establish statistically significant evidence of reduction in duration and presence or absence of adverse outcome as well as benefit
- Follow up studies need to be done to fill up other gaps in knowledge which include difference in the different routes of administration and importantly the pain score/potential analgesic effects of the use of Hyoscine Butyl Bromide which might be sufficient benefit to advocate for its use in addition to the reduction in the duration of labour.
References
- American College of Obstetrics and Gynecology Committee on Practice Bulletin- Obstetrics. Dystocia and Augmentation of labour. Obstet Gynecol. 2003. 102(6):1445-1554.
- Norwitz ER, Robinson JN, Repke JT. Labour and delivery. Gabbe SG, Nebyl JR, Simpson JL. (Eds). Obstetrics: Normal and problem pregnancies. 3rd Ed. New York Churchill Livingstone: 2003.
- Melah GS, El-Nafaty AU, Massa AA, Audu BM. Obstructed labour: a public health problem in Gombe, Gombe State. Niger J Obstet Gynaecol. 2003.(23): 369-373.
- O’Driscoll K, Meagher D. Introduction Active Management of Labour. 2nd Ed. Eastbourne, United Kingdom: Bellaire Tindall. 1986.
- Brown HC, Paranjothy S, Dowswell T, Thomas J. Package of care for active management in labour for reducing caesarean section rates in low-risk women. Cochrane Database of Systematic Reviews. 2013. Issue 9. Art No: Cd004907.
- Neilson JP, Lavender T, Quenby S, Wray S. Obstructed Labour. Br Med Bull. 2003. 67. 191-204.
- Sadler LC, Davison T, Mc Cowan LM. A randomised controlled trial and meta-analysis of active management of labour. BJOG. 2000. 107(7):909-915.
- Mohamel IE, Nuzhet A, Ahmed A. Hyoscine Butyl Bromide for management of prolonged labour. U.S. National Library of Medicine, available at clinicaltrials.gov. 2014.
- Rowher AC, Khondowe O, Young T. Antispasmodics for labour(review), The Cochrane Collaboration. John Wiley and Sons Ltd. 2013.
- World Health Organization recommendation on the use of antispasmodic agents for prevention of delay in labour. The WHO Reproductive Health Library; Geneva: World Health Organization. 2014.
- Labour. Obstetrics by Ten Teachers 18th Ed. Phillip NB(Ed). Book Power. 2006. 220-254.
- Orhue AAE. Normal Labour. Textbook of Obstetrics and Gynecology for Medical Students 2nd Edition. Agboola A(Ed). Heimann Educational Books. 2006. 284-302.
- World Health Organization. Understanding prolonged and obstructed labour. Education material for teachers of midwifery: midwifery education modules. 2nd ed. France: WHO Press; 2008. p. 15-36.
- Allen VM, Baskett TF, O’Connell CM, McKeen D, Allen AC. Maternal and Perinatal Outcomes with increasing duration of the second stage of labour. Obstet Gynecol. 2009. 113(6):1248-1258.
- Hamilton Richart Tarason Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones and Bartlett Learning. 2015. 270.
- WHO Model List of Essential Medicines (19th List). World Health Organization. 2015.
- New Zealand Data Sheet. Buscopan and Buscopan Forte- Hyoscine Butyl Bromide
- Imaralu JO, Olaleye A. Effect of Hyoscine Butyl-Bromide on the duration of active phase of labour: A randomized controlled trial. Taiwanese Journal of Obstetrics and Gynecology. 2017. 56(6):725-730.
- Kjaergaard H, Olsen J, Ottesen B, Dykes AK. Incidence and outcomes of dystocia in the active phase of labour in term nulliparous women with spontaneous labour onset. Acta Obstetricia et gynecologica Scandanavica. 2009. 88(4):402-407.
- Samuels LA, Christie L, Roberts-Gittens B, Fletcher H, Frederick J. The effect of hyoscine butyl bromide on the first stage of labour in term pregnancies. BJOG. 2007. 114(12)
- Serpil K, Osman A, Nezli Y, Begum A, Neslihan B, Rehmat B. Effect of intravenous hyoscine-N-butyl bromide on active phase of labour progress: a randomized double-blind placebo-controlled trial. The Journal of Maternal-Fetal and Neonatal Medicine. 2015. 28(9): 1038-1042.
- Aggarwal P, Zutshi V, Batra S. Role of hyoscine N-butyl bromide (HBB-Buscopan) as labour analgesic. Indian J Med Sci. 2008. 62(5):179-184.
- Al Qahtani A, Al Hajeri F. The effect of hyoscine butyl bromide in shortening the first stage of labour: A double-blind randomised controlled clinical trial. Ther Clin Risk Menog. 2011. 7:495-500.
- Makvandi S, Tadayon M, Abbaspor M. Effect of hyoscine N butyl bromide rectal suppository on labour progress in primigravid women: a randomised double-blind placebo-controlled clinical trial. CMJ. 2011. 52:159.
- Kandil MA, Sayyed TM, El Mallah EM, Rezek MA, Zidan HM. Hyoscine butyl bromide for shortening of the first stage of labour in primigravid women. Menovfia Med J. 2017. 30:350-355.
- Barau D, Agida E, Onafowokan O, Adebayo F. Effect of Hyoscine Butyl Bromide on the course of labour. Open Journal of Obstetrics and Gynecology. 2018. 8:1102-1108.
- Isah AD, Adewole N, Zaman J. A five-year survey of caesarean delivery at a Nigerian tertiary Hospital. Trop J Obstet Gynaecol. 2018. 35:14-7
- Anyaka C, Ocheke A, Shambe I, Egbodo C, Pam V, Karshima J, Daru P. Trends in Elective Caesarean Section at the Jos University Teaching Hospital, Jos, Nigeria. Science Journal of Clinical Medicine. 2016. 5(6)
- Garba JA, Burodo AT, Saidu AD, Sulaiman B, Umar AG, Ibrahim R, Nasir AM. Instrumental vaginal delivery in Usmanu Danfodiyo University Teaching Hospital, Sokoto: A ten-year review. Trop J Obstet Gynaecol. 2018; 35:123-7
- WHO recommendation on duration of the first stage of labour. 2018. Available at extranet.who.int/rhl/topics/preconception-pregnancy-childbirth-and-postpartum-care/care-during-childbirth/care-during-labour-1st-stage/who-recommendation-duration-first-stage-labour.