Biochemical Investigation into the Benefit of Quercetin Supplementation in Hypoxic Mice
Anthony T. Eduviere,A Lily O. Otomewo,B,* Emuesiri G. Moke,A Joshua OnohwosafeA
Abstract
Context: Since the proper flow of oxygen into the human body signifies one of the core features of living organisms, a shortage in supply poses a significant threat to survival.
Objective: This study aimed to investigate the effects of intermittent hypoxia on mice and the benefit of pre-administered quercetin on such effects.
Materials and Methods: Thirty (30) mice were procured and shared into five (5) experimental groups comprising six (6) mice each (n=6). Group I was the normal control group, group II was the negative group and groups III-V were the quercetin groups. Group I and II received distilled water while III-V received quercetin in different doses (10, 20 and 40 mg/kg p.o.). Also, all groups were subjected to the hypoxia protocol except group I. All treatments were carried out for seven (7) days. Afterwards, blood obtained via cardiac puncture, brains and lungs of select mice were respectively subjected to haematological evaluation and intense biochemical analysis. Also, specific lung tissue was subjected to histology using Hematoxylin and Eosin staining procedure.
Results: Quercetin significantly (p<0.05) slowed the rate of possible progression of systemic inflammation by enhancing antioxidant systems in the lungs and brain by increasing glutathione, catalase and superoxide dismutase activity in addition to displaying anti-inflammation by inhibiting the activity of myeloperoxidase. Also, quercetin significantly increased red blood cell and haemoglobin content when compared with the model group.
Conclusion: Quercetin attenuated hypoxia-induced biochemical alterations in major organs of the body which can be accredited to its antioxidative, neuroprotective and anti-inflammatory properties.
Keywords: Blood, Brain, Hypoxia, Lungs, Prooxidants, Antioxidants
1. Introduction
Oxygen is the element at the very heart of aerobic metabolism in mammals. A shortage in its supply, known as hypoxia, is the absence of sufficient volume of oxygen within the cell.1 Hypoxia mostly originates from hypoxemia, dysfunctional oxygen delivery system, and/or impaired oxygen uptake system.1 Since oxygen is regarded fuel for living organisms, high-altitude dwellers at above 3000m may experience progressive damage to the lungs, brain, heart, and other organs due to the insufficiency of oxygen available for inspiration.2-4
Over the years, many preclinical studies have employed various models and techniques in a bid to enlist the physiologic and biochemical effect of hypoxia on selected organs of the human body such as the heart, brain, liver, kidneys, blood, etc, while others investigated the possible benefit of different drugs on these affected organs. In some of those studies, hypoxia significantly induced morphological lesions in specific brain regions concomitant with behavioural/performance deficits, a decline in antioxidant activity,5-7 an increase in leukocyte count which signals inflammation8 and a reduction in haemoglobin content.9
In a bid to contribute to the existing literature on hypoxia, the authors of the current research sought to investigate the biochemical effects of hypoxia on major organs of the body and the possible benefit of pre-administered oral preparations of quercetin using Swiss albino mice.
2. Materials and Methods
2.1 Materials
Quercetin (QCN; 3, 3', 4', 5, 7-pentahydroxyflvanone; Sigma-Aldrich®, St. Louis, MO, USA) was the model drug used in this study. Dimethyl sulfoxide (DMSO) was selected as its solvent.
2.2 Subjects
A total of thirty (30) Swiss albino mice (male; n = 30; wt = 26.0±2.0 g) were used in the present study. They were provided by the animal house of Basic Medical Sciences, Delta State University, Abraka. Afterwards, they were allowed a week of acclimatisation at room temperature. According to the NIH guide for laboratory animals (NIH Publications No. 8023, revised 1978), the mice were exposed to equal hours in the light as well as the dark cycle environments while receiving rodent pellet diet and water ad libitum. All procedures in this experiment were in accordance with the ARRIVE guidelines and also approved by the ethics committee of the faculty.
2.3 Experimental design and treatment groups
At the end of the weeklong acclimatisation period, the mice were randomly divided by alternation into five (5) groups made up of six (6) animals each (n=6). Thereafter, grouping was done based on the substance of administration and exposure to hypoxic stress (HS).
I. Group I mice received vehicle of 10 ml/kg p.o. 5% DMSO and were allowed normal air within the laboratory, thus considered the normal control group;
II. Group II mice also received vehicle p.o. and were exposed to hypoxic stress, thus considered the negative control group;
III. Group III mice received 10 mg/kg quercetin in addition to exposure to hypoxic stress (i.e., QCN 10 mg/kg p.o. + HS);
IV. Group IV mice received 20 mg/kg quercetin in addition to exposure to hypoxic stress (i.e., QCN 20 mg/kg p.o. + HS);
V. Group V mice received 40 mg/kg quercetin in addition to exposure to hypoxic stress (i.e., QCN 40 mg/kg p.o. + HS).
These administrations were carried out daily for seven (7) consecutive days.
Induction of hypoxic stress was achieved by daily locking each mouse in a 250 mL container for 20 mins.10 Note that exposure to the hypoxic stress protocol was done one hour after the drug administration for each day (Fig. 1). Afterwards, on the eighth day, bioassays of the extracted tissues and organs were carried out.
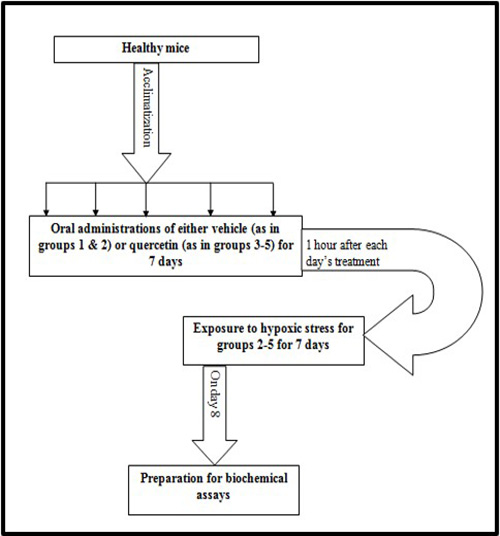
Fig. 1 Experimental schedule
2.4 Collection of blood for haematological assays
Whole blood samples of selected mice from all groups were collected from the exposed heart into heparinized tubes.11 An analysis including white blood cell (WBC) count, red blood cell (RBC) count, haemoglobin (Hb) estimation and other blood volume parameters such as packed cell volume (PCV) were carried out. For more accuracy, these tests were carried out within ~10 min of blood withdrawal.12
2.5 Tissue Preparation for Biochemical assays
Also, selected mice from all groups were euthanized by exposure to chloroform and their respective brain and lungs were harvested. Thereafter, each brain and lung sample was weighed and homogenised in different 10% w/v phosphate buffers (0.1M, pH 7.4). The homogenates were then centrifuged at x104 rpm for 15 min at 4oC. The resulting supernatants were immediately extracted and used for the oxidant activity assays.13
2.5.1 Glutathione (GSH) concentration
To determine GSH concentration in brain and lung tissues, 0.4 ml of 20% TCA was added to 0.4 ml of the individual homogenates and centrifuged using a cold centrifuge at 10,000 rpm for 20min to obtain the supernatants. Following the Beutler method,14 0.25ml of that supernatant was added to 2ml of 0.6mM DTNB to produce 2-nitro-5-thiobenzoic acid which triggers a change in absorbance. This change was measured using a UV/VIS spectrophotometer (Techmel and Techmel® USA) at 412 nm against a blank. The concentrations of GSH in the lung and brain tissues were expressed as µmol/g tissue.
2.5.2 Malondialdehyde (MDA) concentration
The degree of lipid peroxidation, MDA, was approximated using Okhawa’s method.15 A mixture of 0.4 ml of the individual brain and lung supernatants, 1.6 ml of Tris–KCl buffer, and 0.5 ml of 30% TCA was formed. Then, 0.5 ml of 0.75% TBA was added and placed in an 80°C water bath for 45 min. Afterwards, the reaction was cooled and centrifuged for 15 min at 3x103 rpm. The colour of each resulting supernatant was measured with a spectrophotometer at 535 nm and expressed as µmol/g tissue.
2.5.3 Superoxide dismutase (SOD) activity
This was carried out according to Sirota,16 with minimal modifications and SOD activity was expressed as unit/min/mg protein.
2.5.4 Catalase activity
Catalase (CAT) activity was estimated using a modified method described by Luck17 and Aebi.18 The catalase enzyme activity was expressed as µmol of H2O2 decomposed per minute/mg protein.
2.5.5 Nitrite concentration
Brain and lung nitrite concentration was determined using Greiss reagent in line with Giustarini’s study.19 Greiss reagent (100 µl) was added to 100 µl of the individual supernatants and absorbance was measured at 540 nm. The nitrite concentration was therefore estimated from a standard curve obtained from sodium nitrite (0-100 uM).
2.5.6 Myeloperoxidase activity
Lung myeloperoxidase (MPO) activity was determined based on established protocols by Eduviere20 and Gorudko.21 Finally, MPO activity was estimated by adding 0.2 ml of the supernatant to 2.8 ml of a mixture containing 0.167 mg/ ml O-dianisidine in the potassium phosphate buffer and 0.15 mM H2O2. Then, the change in absorbance at 450 nm was monitored over 3 min using UV/VIS spectrophotometer.
2.6 Haematological assay
Red and white blood cells were estimated by simple counting using the haemocytometer as described by Rusia and Sood.22 Also, the packed cell volumes (PCV) and haemoglobin contents were calculated using the microhematocrit method and the cyanmethemoglobin method respectively.23
2.7 Tissue Preparation for Lung Histology
Following euthanization, selected mice from each group were subjected to intracardiac perfusion using phosphate-buffered saline (PBS) and 10% neutral buffered formalin (NBF). Once the lungs were pale in colour, they were excised and tissue sections obtained were further suspended in NBF until paraffin wax embedment. However, they were resuspended in fresh NBF after the first twenty-four hours (24 h). Lung tissues (5–6 µm thick) from each group were processed using the routine method for paraffin wax embedment in preparation for histology and fixed on glass slides. The Hematoxylin and Eosin stain was administered to the paraffin wax embedded sections for cell quantification24 and the sections were subjected to microscopy and digital photography. Also, neuronal density of each section was estimated by simple neuronal counts from the photomicrographs.
2.8 Statistical analysis
All experimental data obtained were presented as Mean ± standard deviation (SD). Result analysis was done using one way analysis of variance (ANOVA) followed by Bonferroni’s Multiple Comparison test. Graph Pad InStat® Biostatistics software was also used to determine the level of significance for all tests which was set at p <0.05.
3. Results
3.1 Effect of quercetin on haematological alterations in hypoxic mice
The blood of group II mice which were exposed to only hypoxia revealed significant (p<0.05) changes to important blood parameters – an increase in WBC count, a decrease in RBC count, Hb content (Table 1), and also a decrease in PCV (Fig. 2) – when compared to the normal control group I (F(4,25)=10.02; p<0.0001). In all tests, the quercetin groups III-V recorded significant (p<0.05) reversal of these alterations.
Table 1: Effect of quercetin on lung oxidative profile in hypoxic mice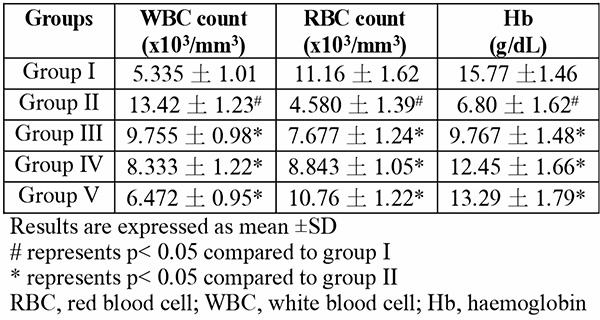
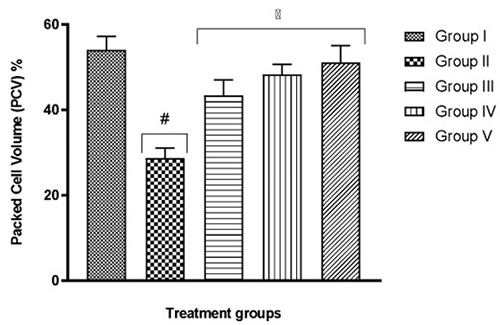
Fig. 2 Effect of quercetin on blood cell volume of hypoxic mice
Results are expressed as mean ±SD
# represents p< 0.05 compared to group I
* represents p< 0.05 compared to group II
3.2 Effect of quercetin on lung oxidative activity in hypoxic mice
The lung tissue of group II mice which were exposed to only hypoxia revealed significant (p<0.05) changes to important lung oxidants – an increase in MDA content, a decline in SOD activity, CAT activity, GSH content (Table 2), and also an increase in MPO activity and nitrite level (Fig. 3 and 4, respectively) – when compared to the normal control group I (F(4,25)=12.13; p<0.0001) . In all tests, the quercetin groups III-V recorded significant (p<0.05) reversal of these alterations.
Table 2: Effect of quercetin on lung oxidative profile in hypoxic mice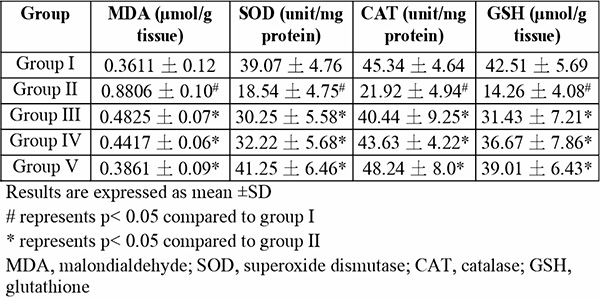
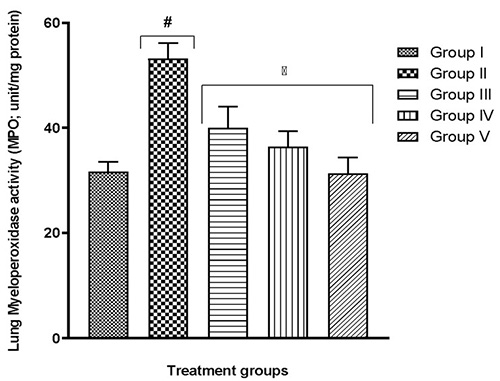
Fig. 3 Effect of quercetin on lung myeloperoxidase activity in hypoxic mice
Results are expressed as mean ±SD
# represents p< 0.05 compared to group I
* represents p< 0.05 compared to group II
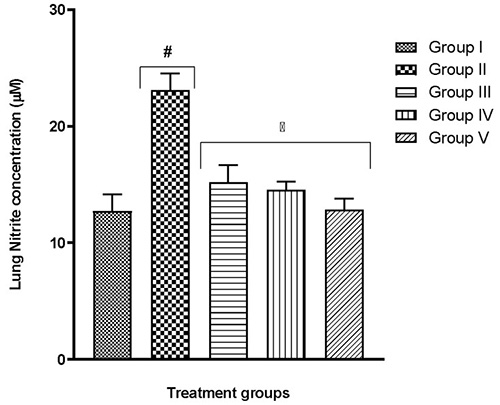
Fig. 4 Effect of quercetin on lung nitrite concentration in hypoxic mice
Results are expressed as mean ±SD
# represents p< 0.05 compared to group I
* represents p< 0.05 compared to group II
3.3 Effect of quercetin on brain oxidative activity in hypoxic mice
Tissues from selected brain regions of group II mice which were exposed to only hypoxia revealed significant (p<0.05) changes to biologically-active brain antioxidants (Table 3) and prooxidants (Fig. 5 and 6) – a reduction in GSH content, a decline in SOD and CAT activity, as well as an increase in MDA and nitrite levels – when compared to the normal control group I (F(4,25)=29.37; p<0.0001, (F(4,25)=14.73; p<0.0001)). In all tests, the quercetin groups III-V recorded significant (p<0.05) reversal of these alterations.
Table 3: Effect of quercetin on brain antioxidants in hypoxic mice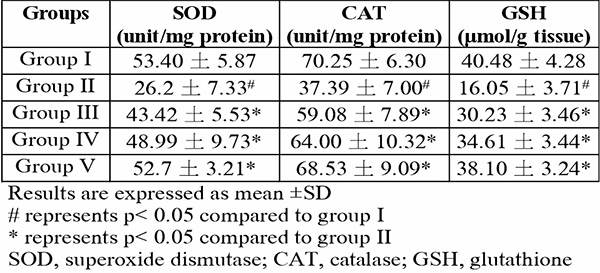
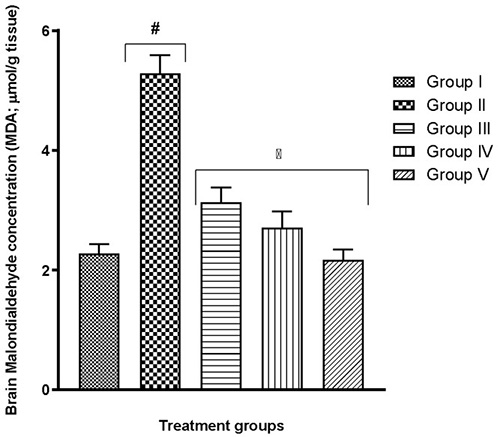
Fig. 5 Effect of quercetin on brain prooxidant level in hypoxic mice
Results are expressed as mean ±SD
# represents p< 0.05 compared to group I
* represents p< 0.05 compared to group II
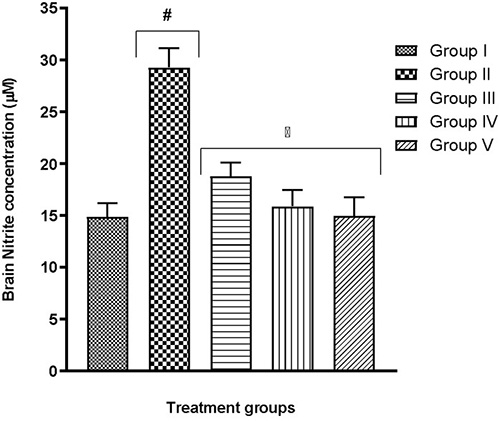
Fig. 6 Effect of quercetin on brain nitrite level in hypoxic mice
Results are expressed as mean ±SD
# represents p< 0.05 compared to group I
* represents p< 0.05 compared to group II
3.4 Effect of quercetin on lung histology in hypoxic mice
As shown in Fig. 7, alveolar epithelial cells and pulmonary capillaries responsible for tissue perfusion of the hypoxia only group (group II) were significantly damaged when compared to the normal control (group I). This could be linked to increased lung inflammation as evidenced in the increase of MPO and prooxidant activity (Fig 3 and Table 2) due to hypoxia. However, significant tissue repair and resolution of inflammatory processes was observed in groups that received quercetin as the alveolar cells were protected from inflammation-induced apoptosis and alveoli damage.
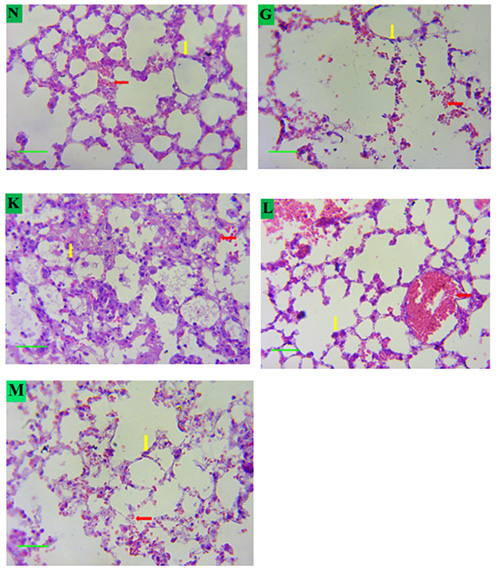
Fig. 7 Photomicrograph showing the effect of quercetin on the histology of the lung alveoli tissue in mice exposed to hypoxic stress
Key:
N – Group I.
G – Group II.
K – Group III.
L – Group IV.
M – Group V.
Red arrow: Pulmonary capillaries. Yellow arrow: Nuclei
Magnification: X400.
4. Discussion
Hypoxia is a common experimental model of physiological stress. As with all other stressors, there are pre-programmed sensors within the human body with instructions to induce appropriate responses to stress. The two most powerful responders are the sympatho-adrenomedullary (SAM) and the hypothalamic-pituitary-adrenal (HPA) axes.25,26 Over the years, low oxygen supply has been considered a major threat to the normal physiologic wellbeing of organisms. As a matter of fact, a wide range of conditions have been linked to hypoxia in previous studies: cardiac arrest, seizures, arrhythmia, respiratory diseases,27 anxiety disorders, depression and mood changes.28 In this current study, the authors sought to investigate the simple biochemical changes that occur in the vital organ systems of mice using an intermittent respiratory hypoxia model. Therefore, the level and activity of some reactive oxygen species (ROS) and enzymes in the lungs and brain were estimated as well as the levels of relevant blood constituents.
More recent studies proceeded to link a shortage in oxygen supply to an increase in the generation of ROS.29-39 Although these molecules are useful in particular conditions, higher levels might result in an increase in lipid peroxidation.29,40 As a result, malondialdehyde (MDA) production may become excessive thereby frustrating the efforts of defensive enzymes superoxide dismutase (SOD) and catalase (CAT). This is evident in the present study where the highest MDA and nitrite levels were seen in the group exposed to hypoxia alone (Table 2, Fig. 4-6). It is also evident that the defence enzymes CAT, SOD and GSH in the brain and lungs were diminished in the hypoxia-only group (Table 2, Table 3). Similar to MDA, the activity of MPO was also significantly elevated in the lungs of the hypoxia-only group (Fig. 3 and 4). This increase in MPO activity in the lungs is most likely an indicator of the outset of inflammation as previously postulated that hypoxia can cause inflammation.41
According to Yoon and Ponka,42 hypoxia is an important stimulus for the secretion of erythropoietin, a precursor for red blood cell (RBC) production. A previous study on hypoxia revealed that acute hypoxia increases erythropoietin levels which subsequently increase RBC production, packed cell volume (PCV), haemoglobin (Hb) concentration and total blood volume.43-45 This does not correlate with the present study which studied the effect of a seven-consecutive day exposure to hypoxia. Here, chronic hypoxia led to a significant increase in white blood cells (WBC) which could mean inflammation just like MPO, and a decrease in RBC count, Hb concentration and PCV.
Finally, the results from this present study generally revealed that chronic hypoxia can cause damage to the central nervous system, the pulmonary and circulatory systems by encouraging oxidative stress and inflammation. However, this present study also revealed that pre-treatment with quercetin slowed the whole process of damage significantly and opposed specific system deterioration. Quercetin exhibited its anti-inflammatory activity by opposing the activity of lung MPO and excessive production of WBCs in the quercetin-treated groups.46 These effects were further confirmed by the histological analysis of the lung alveoli tissues which described a role of quercetin in alleviating the inflammation-induced alveoli damage induced by hypoxia.47 Also, its neuroprotective and antioxidative activity were exhibited by potentiating the production of antioxidative defence enzymes GSH, CAT and SOD in the quercetin-treated groups.48 Blood constitution of the quercetin-treated groups was brought towards normal through, what the authors believe is, the hepatoprotective effect of quercetin since erythropoietin secretion is done by the kidneys.49 The clinical implication of these findings is that quercetin consumption from various sources must be encouraged in individuals who often indulge in activities or suffer from diseases that may limit their oxygen supply as a means to improve their health regardless.
In conclusion, quercetin possesses the ability to counter the adverse effects of hypoxia across the various body systems.
Declaration of interests
The authors declare that they have no conflicts of interest.
Funding
None
Ethical statement
This research was approved by the institutional animal care and use committee (REF/FBMS/DELSU/21/105)
References
- MacIntyre NR. Tissue hypoxia: implications for the respiratory clinician. Respir Care 2014;59(10):1–7. Accessed via DOI: 10.4187/respcare.03357
- Young AJ, Berryman CE, Kenefick RW, et al. Altitude acclimatization alleviates the hypoxia-induced suppression of exogenous glucose oxidation during steady-state aerobic exercise. Front Physiol 2018; 9(830): 1-13. Accessed via https://doi.org/10.3389/fphys.2018.00830
- Gonzalez Garay A, Molano Franco D, Nieto Estrada VH, Martí-Carvajal AJ, Arevalo-Rodriguez I. Interventions for preventing high altitude illness: Part 2. Less commonly-used drugs. Cochrane Database Syst Rev 2018;3: CD012983.
- Paul S, Gangwar A, Bhargava K, Khurana P, Ahmad Y. Diagnosis and prophylaxis for high-altitude acclimatization: Adherence to molecular rationale to evade high-altitude illnesses. Life Sci 2018;203:171-176.
- Zhang X, Rui L, Wang M, Lian H, Cai L. Sinomenine attenuates chronic intermittent hypoxia-induced lung injury by inhibiting inflammation and oxidative stress. Med Sci Monit 2018;24:1574-1580.
- Wu H, Luo D, Li C, Zhang H, Shunxian A, Zhang Y, Sun C. Chicoric acid improves heart and blood responses to hypobaric hypoxia in tibetan yaks. Am J Chin Med 2018;46:339-355.
- Nakanishi K, Tajima F, Nakamura A, et al.. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J Physiol 1995;489(3):869-876
- Esteva S, Pedret R, Fort N, Torrella JR, Pagčs T, Viscor G. Oxidative stress status in rats after intermittent exposure to hypobaric hypoxia. Wilderness & Environmental Medicine 2010;21:325–331
- Yang S, Yan T, Wu H, et al. Acute hypoxic stress: effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides). Fish and Shellfish Immunology 2017. Accessed via doi: 10.1016/j.fsi.2017.06.035.
- Eduviere AT, Otomewo LO. Minocycline abrogates lung oxidative damage and haematological perturbations in mice exposed to hypoxia. Journal of Drug Delivery and Therapeutics 2022;12(1-S):82-90
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. Journal of Pharmacology & Pharmacotherapeutics 2010;1(2):87-93
- Ajonijebu DC, Olayanju AO, Eduviere AT, et al. Effects of Calcitriol supplementation on the hematological parameters of sleep deprived Wistar rats. International Journal of Health Sciences and Research 2016;6(3):1-8 ISSN 2249-9571
- Parrilla-Taylor DP, Zenteno-Savín T. Antioxidant enzyme activities in Pacific white shrimp (Litopenaeus vannamei) in response to environmental hypoxia and reoxygenation. Aquaculture 2011;318:379–383
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61: 882–888.
- Okhawa H, Ohishi N, Yogi K. Assay for lipid peroxidation in animal tissue by TBA reaction. Anal. Biochem. 1979;95:35–38.
- Sirota TV. Standardization and regulation of the rate of the superoxide-generating reaction of adrenaline autoxidation used for evaluation of pro/antioxidant properties of various materials. Biochemistry (Moscow), Supplement Series B: Biomedical Chemistry 2017;11:128-133
- Lück H. Catalase. Methods Enzymol 1984;105:121–126.
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:457–464
- Giustarini D, Rossi R, Milzani A, Dalle-Donne I. Nitrite and nitrate measurement by Greiss reagent in human plasma: evaluation of interferences and standardization. Methods Enzymol 2008;440:361-380.
- Eduviere AT, Umukoro S, Adeoluwa OA, Omogbiya IA, Aluko OM. Possible mechanisms involved in attenuation of lipopolysaccharide-induced memory deficits by methyl jasmonate in mice. Neurochem Res 2016;41:3239-3249
- Gorudko IV, Tcherkalina OS, Sokolov AV, et al. New approaches to the measurement of the concentration and peroxidase activity of myeloperoxidase in human blood plasma. Russian Journal of Bioorganic Chemistry 2009;35:566-575
- Rusia V, Sood SK. Routine hematological tests. Medical laboratory technology 1992;1:252-258
- Nelson DA, Morris MW. Basic methodology. Hematology and coagulation, part IV. In: Nelson, D.A., Henry, J.B. (Eds.), Clinical Diagnosis and Management by Laboratory Methods, 17th ed. Saunder Company, Philadelphia, USA, 1989; pp. 578–625 (Chapter 27).
- Nagato AC, Bezerra FS, Lanzetti M, et al. Time course of inflammation, oxidative stress and tissue damage induced by hyperoxia in mouse lungs. International Journal of Experimental Pathology 2012;93(4):269-278
- Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front. Behav. Neurosci 2018;12(127):1-23
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 2000;21:55–89.
- Maurer J, Rebbapragada V, Borson S, et al. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest 2008;134:43s–56s.
- Bahrke MS, Shukitt-Hale B. Effects of altitude on mood, behaviour and cognitive functioning. A review. Sports Med 1993;16:97–125.
- Xing G, Qualls C, Huicho L, et al. Adaptation and maladaptation to ambient hypoxia; Andean, Ethiopian and Himalayan patterns. PLOS One 2008;3(6):e2342.
- Bakonyi T, Radak Z. High altitude and free radicals. J. Sports Sci Med. 2004;3(2):64–69.
- Magalhăes J, Ascensăo A, Viscor G, et al. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat Space Environ Med. 2004;75(1):16 –22.
- Marin-Corral J, Fontes CC, Pascual-Guardia S, et al. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid Redox Signal. 2010;12(3):365–380.
- Moller P, Loft S, Lundby C, Olsen NV. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J 2001;15(7):1181–1186.
- Askew EW. Work at high altitude and oxidative stress: antioxidant nutrients. Toxicology 2002;180(2):107–119.
- Subudhi AW, Jacobs KA, Hagobian TA, et al. Antioxidant supplementation does not attenuate oxidative stress at high altitude. Aviat Space Environ Med. 2004;75(10):881– 888.
- Hou Y, Wang X, Chen X, et al. Establishment and evaluation of a simulated high-altitude hypoxic brain injury model in SD rats. Molecular Medicine Reports 2019;19:2758-2766
- Zhang X, Rui L, Wang M, Lian H, Cai L. Sinomenine attenuates chronic intermittent hypoxia-induced lung injury by inhibiting inflammation and oxidative stress. Med Sci Monit 2018;24: 1574-1580.
- Yang G, Min D, Yan J, Yang M and Lin G: Protective role and mechanism of snakegourd peel against myocardial infarction in rats. Phytomedicine 2018;42: 18-24.
- Wu H, Luo D, Li C, et al. Chicoric acid improves heart and blood responses to hypobaric hypoxia in tibetan yaks. Am J Chin Med 2018;46: 339-355.
- Bailey DM, Davies B, Young IS. Intermittent hypoxic training: implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin Sci. 2001;101(5):465–475.
- Eduviere AT, Otomewo LO, Egerega EV, Charles EO. Effect of verapamil on haematological parameters and lung oxidative profile in mice subjected to hypoxia. Int. J. Forensic Med. Invest. 2022;8(1):1-7
- Yoon D, Ponka P, Prchal JT. Hypoxia. 5. Hypoxia and hematopoiesis. Am J Physiol Cell Physiol 2011;300(6):C1215-C1222
- Eckardt KU, Boutellier U, Kurtz A, Schopen M, Koller EA, Bauer C. Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J Appl Physiol. 1989;66(4):1785–1788.
- Ferretti G, Boutellier U, Pendergast DR, et al. IV. Oxygen transport system before and after exposure to chronic hypoxia. Int J Sports Med. 1990;11(S1):S15–S20.
- Rodriguez FA, Casas H, Casas M, et al. Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity. Med Sci Sports Exerc. 1999;31:264 –268.
- Tripathi A, Kumar B, Kumar M, Kaur P, Sagi SSK. Quercetin prophylaxis: a novel approach to prevent hypoxia-mediated increase in oxidative stress, PDE-5 activity and transvascular leakage in the lungs of rats. HSOA J Pulm Med Respir Res 2020;6(2):1-29.
- Hohmann MS, Habiel DM, Coelho AL, Verri WA Jr, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol 2019;60(1):28-40
- Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019;24(6):1123
- Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013;27:41