Premalignant features of the prostate can be ameliorated by Uvaria chamae root extract: Modeling prostate cancer with cadmium and alternative treatment option in adult Wistar rats
Gabriel D. Edem1*, Christopher L. Sakpa2, Vitalis C. Ezeuko2
Abstract
Background: Prostate cancer is the most commonly diagnosed cancer and the leading cause of cancer deaths in males worldwide. Mortality attributed with prostate cancer is strongly associated with the age of an individual with relatively higher incidence noticed among aged males.
Aim: The present study was carried out to assess the ameliorative effects of Uvaria chamae root extract on cadmium-induced carcinogenic potentials in adult male Wistar rats.
Materials and Methods: Sixty (60) adult male rats weighing between 200-210g were used in the current study. They were randomly divided into 10 groups of 6 animals per group (n=6). Group 1 received water and diet alone, group 2 received 3mg/kg of cadmium alone once weekly for 28days. Group 3-6 received 3mg/kg of cadmium for 28days followed by 150mg/kg of casodex, 2500mg/kg, 1500mg/kg and 1000mg/kg of Uvaria chamae root extract respectively for 28days. Group 7 received 150mg/kg of casodex alone for 28days. Group 8-10 received 2500mg/kg, 1500mg/kg and 1000mg/kg of Uvaria chamae root extract alone for 28days. The animals were sacrificed after the last dose schedule, blood sample taken for hormonal assay and prostate tissues were preserved in 10% buffered formalin for histology.
Results: Cadmium was found to raise the serum levels of testosterone, FSH, LH, prolactin and estradiol. However, administration of different doses of the root extract of Uvaria chamae decreased the serum levels of the hormones. Administration of 3mg/kg of cadmium alone caused appearance of histological features including small and crowded glands, undulating acini with contours and rigid lumen, nuclear atypia, nuclear hyperchromasia and infiltration of inflammatory cells. These histological features are often encountered in premalignant lesions of the prostate gland. The different doses of the root extract of Uvaria chamae was able to ameliorate the carcinogenic potentials exerted by cadmium on prostate tissues. These ameliorative changes were conspicuous in 2500mg/kg and 1500mg/kg treated groups respectively when compared to control and standard.
Conclusion: This investigation suggests that, the root extract of Uvaria chamae possesses strong cancer ameliorative tendencies through hormonal regulation. Further research should be conducted to isolate the bioactive ingredients responsible for this important therapeutic ability of Uvaria chamae for the sole purpose of anti-cancer drug production.
Keywords: Prostate cancer, Uvaria chamae, Testosterone, Prolactin, LH, FSH, Estradiol
Introduction
Cancer is an uncontrolled growth of cells resulting in phenotypic expression including invasion and metastasis. Cancer can develop in several ways including DNA damage, DNA mutation or loss of regulatory molecules in the cell cycle.1 Prostate cancer is the fifth leading cause of cancer deaths in men worldwide.2-3 In 2020, a total of about 1,414,249 newly diagnosed cases and 375,000 deaths worldwide have been reported annually.2-3 During prostate carcinogenesis, there are no initial or early symptoms associated with it in most cases, however, late symptoms may include bone pain, weakness of the body due to anemia, paralysis from spinal metastases and renal failure due to urinary tract obstruction. Prostate cancer cases are generally elevated in developed countries and less common in Asia.4 According to the World Health Organization, the following countries have been reported with the highest incidence of cancer, they include: Ireland, Barbados, Estonia, France, Sweden, Bahamas, Puerto Rico, St. Lucia and Martinique and the highest mortality rate was reported in Zimbabwe, Haiti, Zambia, Jamaica, Bahamas and Grenada. Prostate cancer is very uncommon in young men below 45years of age although the incidence is increasing in some developing countries.5 It has been documented that more than 80% of men above 65 years of age tends to develop prostate cancer.4 Mortality statistics associated with prostate cancer can be ethnic dependent with the highest incidence and mortality rate recorded in African-American.3 The risk of developing or dying from prostate cancer is in the ratio of about 1:6 in African-America when compared to 1:8 in the whites.6 Prostate cancer primarily develops in the peripheral zone and this portion of the prostate can easily be palpated via digital examination of the rectum.7 It is an adenocarcinoma that develops particularly from the glandular epithelium. As the cancer cells grow and started proliferate, it invades the surrounding prostate tissue leading to the formation of tumour nodule which can metastasize or may be localized within the prostate for years.8 Prostate cancers are heterogenous with respect to presentation and morphology and nearly all are grouped as adenocarcinoma. The incidence of prostate cancer is directly related to age.6 However, in some men, prostate cancer is slow growing and may not be detected early enough to prevent mortality. Moreso, the peptide hormone, insulin growth factor has been implicated in the biology of prostate cancer.9 Several risk factors such as smoking have been implicated to play a critical role in the progression of prostate cancer. Chemicals from tobacco-smoking can bind to DNA to exert their mutagenic action. Studies have shown that, an elevated level of androgens in smokers could increase the risk of prostate cancer or may contribute to the progression of cancer.10 A study by Huncharek et al. (2010)11 documented an association between smoking and prostate cancer. In their study involving about 2157 prostate cancer cases, they noticed an increased risk of prostate cancer in smokers. Cadmium is a metal with soft, ductile, silvery white, lustrous and electropositive properties. About 70% of cadmium is estimated to be used in construction, mining and refining industries. Human exposure to cadmium are attributed to these sources.12-13 Exposure of cadmium compounds to humans has created a serious health issue such as prostate cancer. Cadmium toxicity may be considered multi-directional, cadmium ions possess a high affinity for biological compounds with sulfhydryl–SH groups such as glutathione GSH and cysteine as well as compounds with disulfide –S-S groups such as reduced glutathione GS-SG thereby resulting in their functional disturbance.14 For centuries, medicinal plants have been employed to treat and manage different cadre of diseases15, one of such plant is Uvaria chamae. It is commonly known as finger root, native to Tropical West and Central Africa where it grows in wet and dry forests and coastal shrub lands.16-17 Despite the use of Uvaria chamae in alternative medicine to treat and manage several disease conditions, a lot still needs to be done in terms of understanding specific active constituents of the plant, hence the rationale behind undertaking this study. This study was designed to evaluate the ameliorating effects of Uvaria chamae in cadmium-induced carcinogenic potentials in adult male Wistar rats.
Materials and methods
Ethical clearance
Ethical clearance was sought and obtained from the Ethical Committee of the School of Basic Medical Sciences, University of Benin, Benin City. Approval number CMS/REC/2022/275 was assigned.
Plant collection and identification
Uvaria chamae root was sourced from a thick bush in Ikot Efre Itak, a local community in Ikono Local Government Area of Akwa Ibom State. The plant was taken to the Department of Plant Biology and Biotechnology, University of Benin for identification and authentication by a Plant Biologist. Herbarium number UBH-U353 was assigned to the plant.
Preparation of plant extract
The roots were excavated from the soil, washed and cut into sizeable pieces. About 500g of the roots were air-dried for seven (7) days, pulverized with the use of electric blender and macerated in 70% ethanol for 72 hours. The solution was filtered and taken to water bath to dry at 450C. Obtained dry matter was sieved and stored in the refrigerator using an air-tight container for further analysis. 157g of the extract was obtained.
Experimental set up
Sixty (60) adult male Wistar rats weighing between 200-210g were purchased and bred at the animal house of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin City. The animals were housed under proper and standard condition, put in clean cages and maintained at room temperature. The animals were given water and standard feed at will and divided into 10 groups (n=6). Below is the treatment regimen for the experimental animals.
Animal sacrifice
At the conclusion of administration regimen, the rats were subjected to chloroform inhalation and sacrificed immediately. A vertical incision from the abdomen to the pelvis was employed to access the prostate gland. Blood sample was taken from the inferior vena cava for hormonal assay.
Histological studies
Prostate tissues were immediately taken and preserved in 10% buffered formalin solution for histology.
Photomicrography
After tissue processing, the processed tissues were viewed under the light microscope (Primo star 415500-0057000) and photomicrographs from experimental and control groups were taken at x100 and x400 magnifications respectively using the microscope's camera (PDV 010-82613119) attached to a computer.
Statistical analysis
Data obtained from this study were analyzed using Graphpad Prism statistical package (version8.0.2). Analysis of Variance (ANOVA) was used to compare mean between groups. All data were expressed as mean ± standard error of mean (S.E.M) at p< 0.05.
Results
Table 1: Treatment regimen for experimental animals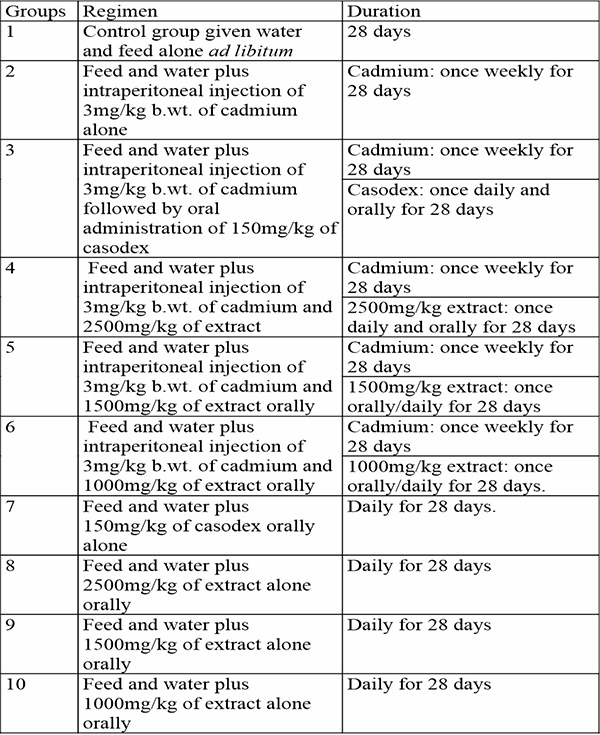
Effect of Uvaria chamae on hormonal changes following cadmium-induced carcinogenic potentials
Cadmium caused a significant increase in the serum level of testosterone (p<0.05) when compared to control and other groups. Administration of Uvaria chamae root extract at various doses significantly reduced serum testosterone levels (p<0.05) when compared to the standard (casodex), control and group 2. Interestingly, an increase was observed in the serum level of estradiol (p<0.05) when compared to control and other groups. Serum levels of progesterone was slightly increased in cadmium treated group but significantly not different across all other treated groups (p<0.05). Similarly, cadmium significantly increased the serum level of luteinizing hormone (p<0.05) when compared to control and other groups. A dose dependent decrease in the serum levels of LH was observed after the administration of different doses of Uvuria chamae root extract when compared to control and the standard (p<0.05). FSH level was significantly increased after cadmium administration when compared to control and other groups (p<0.05) but was significantly reduced in the Uvaria chamae treated groups when compared to control and standard (p<0.05). Moreover, an increase was observed in the serum level of prolactin in cadmium alone treated group (p<0.05) when compared to control and other groups.
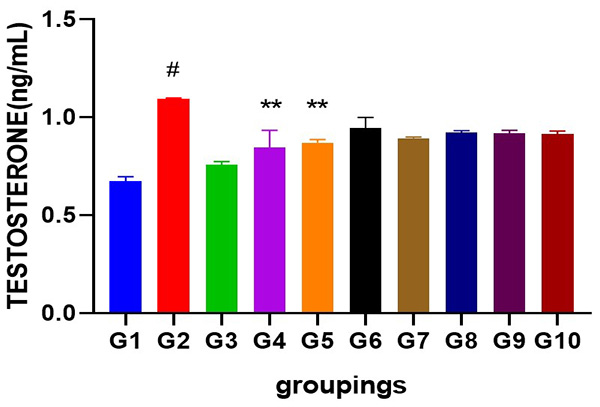
Figure 1: Showing serum testosterone level following cadmium-induced carcinogenic potentials.
# = significantly different from control and other groups @p<0.05
** =significantly different from group 2 and standard @p<0.05
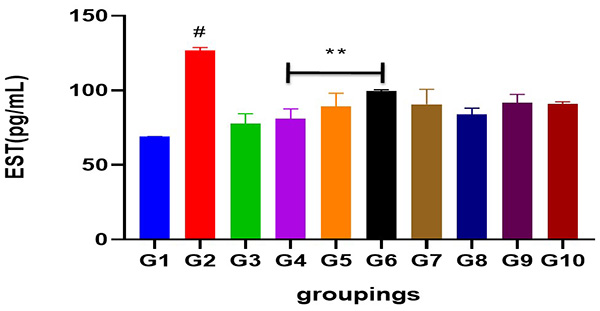
Figure 2: Showing serum estradiol level following cadmium-induced carcinogenic potentials.
# = significantly different from control and other groups @p<0.05
** =significantly different from group 2 and standard @p<0.05
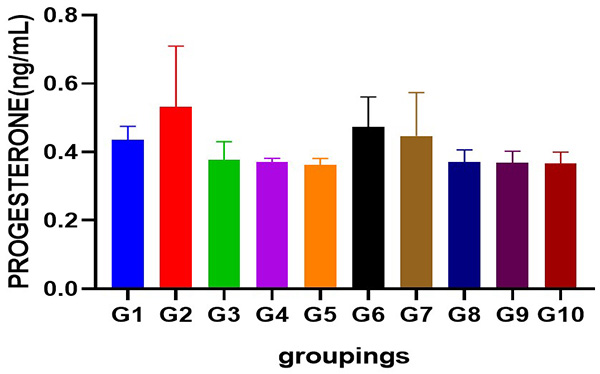
Figure 3: Showing serum progesterone level following cadmium-induced carcinogenic potentials.
p<0.05 not significant across groups
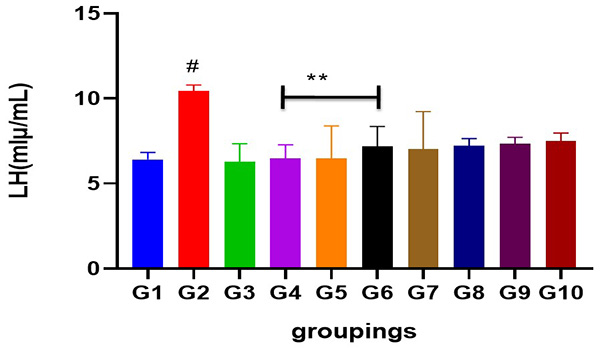
Figure 4: Showing serum LH level following cadmium-induced carcinogenic potentials.
#= different from control and other treated groups
** = different from group 2 when compared to control and standard
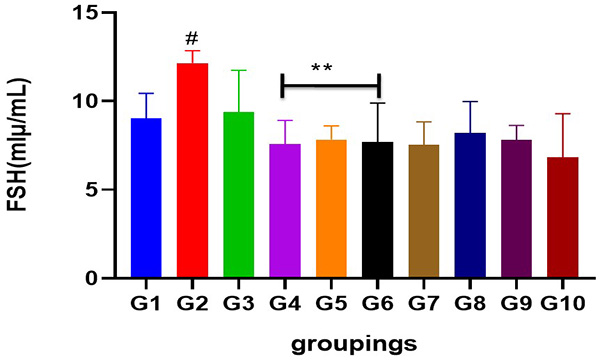
Figure 5: Showing serum FSH level following cadmium-induced carcinogenic potentials.
# = different from control and other treated groups
** = different from group 2 when compared to control and standard
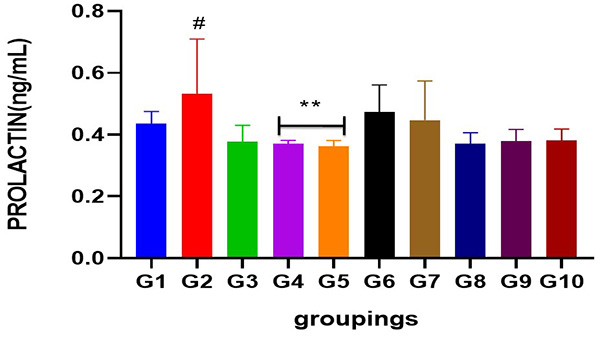
Figure 6: Chart indicating serum prolactin level following cadmium-induced carcinogenic potentials.
# = Significantly different from control and other treated groups
** = Significantly different from group 2 when compared to control
H&E general demonstration of premalignant features of the prostate
Results obtained from H&E revealed the presence of normal prostate epithelial and basal cell layers as well as a well-defined fibromuscular stroma (figure 7A/7B). Interestingly, the following histologic features were noted in cadmium alone treated group; luminal undulation with branching, small and crowded glands with irregular and rigid contour, nuclear atypia, particularly noted was nuclei pleomorphism, nuclear hyperchromasia and infiltration of inflammatory cells in the fibromuscular stroma (figure 8A/8B). Moreso, there was improved lumen without undulation, intact basal cells in many areas, absence of inflammatory cells in the lumen but with penetrating cells in the stroma, all these were noted in the group that was given 3mg/kg of cadmium followed by daily administration of 150mg of casodex respectively for 28days respectively compared to control (figure 9A/9B). Administration of 2500mg/kg of Uvaria chamae root extract after the administration of 3mg/kg of cadmium for 28days showed characteristic features including presence of well-defined acini without undulation and branching, normal lumen without concretions, intact luminal and basal cells and a thin fibromuscular stroma (figure 10A/10B). Similar features were observed in the group administered with 3mg/kg of cadmium and 1500mg/kg of Uvaria chamae root extract respectively for 28days when compared to control and group 2 (figure 11A/11B). Here, there was improved acini, intact lumen without concretions, normal basal and luminal cells as well as well-defined fibromuscular stroma.
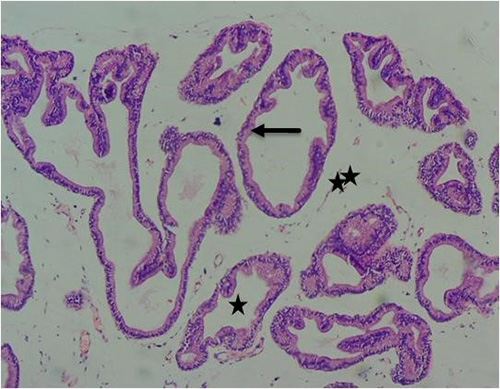
Figure 7A: Photomicrograph of control group showing normal acinar (long arrow), normal lumen (star) and normal surrounding stroma tissue (double star) (H&E) x100 magnification
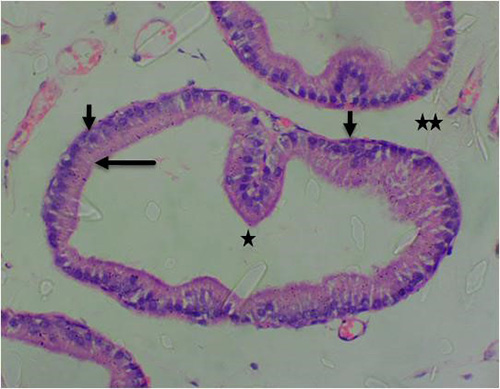
Figure 7B: Photomicrograph of control group showing normal acinar with luminal epithelium (long arrow), normal basal cells (short arrow), normal lumen (star) and normal surrounding stroma tissue (double star) (H&E) x400 magnification
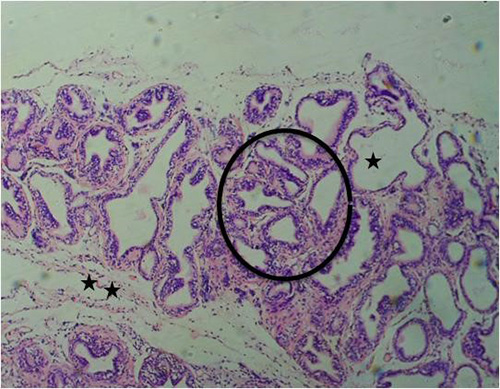
Figure 8A: Photomicrograph of group 2 rats given 3mg/kg of cadmium alone once weekly for 28days showing luminal undulation with branching (star), infiltration of inflammatory cells in the fibromuscular stroma (double star), crowded and small glands with irregular contour/rigid lumen (circle) (H&E) x100 magnification
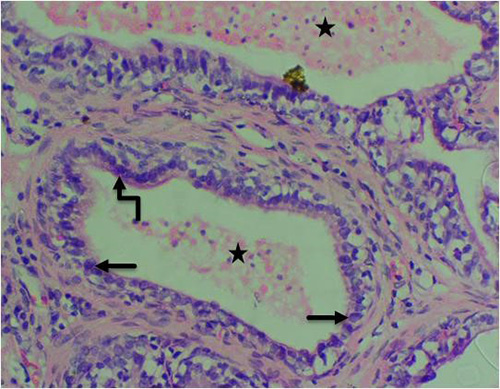
Figure 8B: Photomicrograph of group 2 rats given 3mg/kg of cadmium alone once weekly for 28days showing nuclear atypia (pleomorphism) (arrow), nuclear hyperchromasia (curved arrow) and pink amorphous secretions in the lumen (star). (H&E) x400 magnification
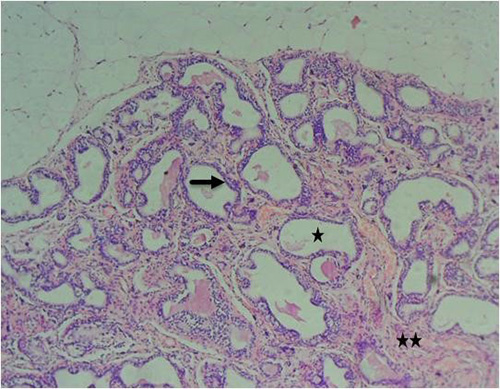
Figure 9A: Photomicrograph of group 3 given 3mg/kg of cadmium plus 150mg/kg of casodex for 28days respectively showing improved acini (black arrow), absence of inflammatory cells into the lumen (star) and fibromuscular stroma with penetrating and proliferating cells (double stars) (H&E) x100 magnification
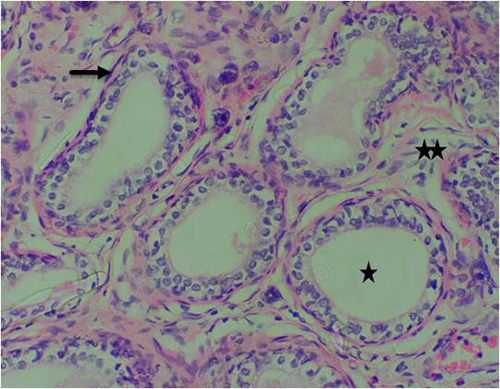
Figure 9B: Photomicrograph of group 3 given 3mg/kg of cadmium plus 150mg/kg of casodex for 28days respectively showing improved lumen (star), intact basal cells in many areas (arrow) and infiltration of inflammatory cells in the stroma (double stars), (H&E) x400 magnification
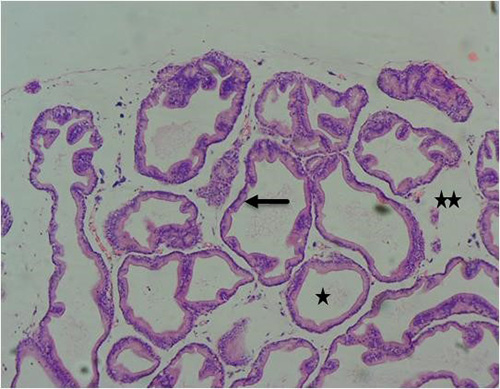
Figure 10A: Photomicrograph of group 4 given 3mg/kg of cadmium plus 2500mg/kg of Uvaria chamae root extract for 28days respectively showing well-defined acini without undulation and branching(arrow), normal lumen (star) and fibromuscular stroma (double stars) (H&E) x100 magnification
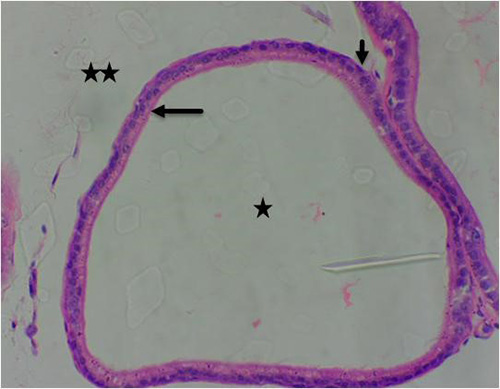
Figure 10B: Photomicrograph of group 4 given 3mg/kg of cadmium plus 2500mg/kg of Uvaria chamae root extract for 28days respectively showing well-defined acini with luminal cells, (long arrow), basal cells (short arrow), normal lumen without concretions (star) and fibromuscular stroma (double stars) (H&E) x400 magnification
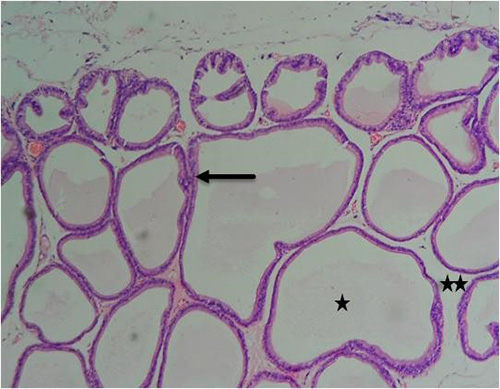
Figure 11A: Photomicrograph of group 5 rats given 3mg/kg of cadmium plus 1500mg/kg of Uvaria chamae root extract for 28days respectively showing improvement in the acini (arrow), intact lumen (star) and fibromuscular stroma (double stars) (H&E) x100 magnification
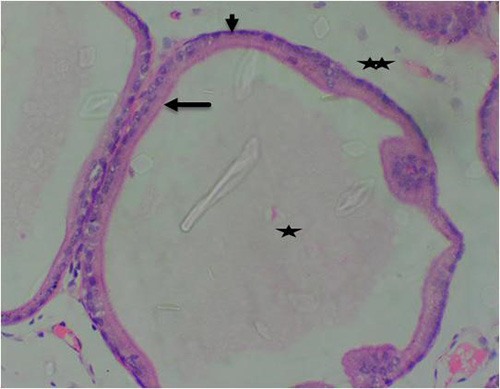
Figure 11B: Photomicrograph of group 5 rats given 3mg/kg of cadmium plus 1500mg/kg of Uvaria chamae root extract for 28days respectively showing improvement in the acini with luminal cells (arrow), basal cells (short arrow), intact lumen without concretions (star) and fibromuscular stroma (double stars) (H&E) x400 magnification
A slight difference was observed in group 6 given 3mg/kg of cadmium followed by 1000mg/kg of Uvaria chamae root extract. The changes observed here include, crowded glands, focal atropy, undulating glands, nuclear atypia particularly nuclei pleomorphism, derangement of luminal cells and fibro-collagenous stroma with infiltration of inflammatory cells (figure 12A/12B). There were no histopathological changes in the prostate tissues of groups 7 (50mg/kg of casodex alone), 8 (2500mg/kg of Uvaria chamae alone), 9 (1500mg/kg of Uvaria chamae alone) and 10 (1000mg/kg of Uvaria chamae alone) respectively (figures 13A/B- 16A/B).
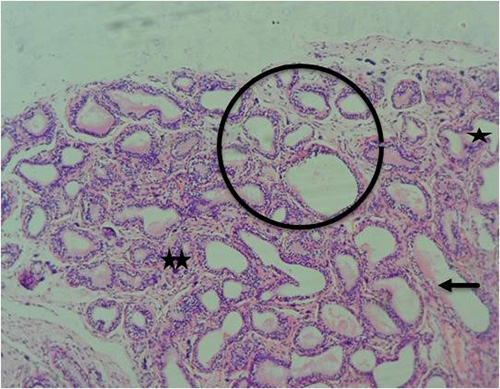
Figure 12A: Photomicrograph of group 6 rats given 3mg/kg of cadmium plus 1000mg/kg of Uvaria chamae root extract for 28days respectively showing focal atropy(arrow), small/crowded glands(circle), undulating gland(star) and fibrocollagenous stroma with infiltrating cells(double stars) (H&E) x100 magnification
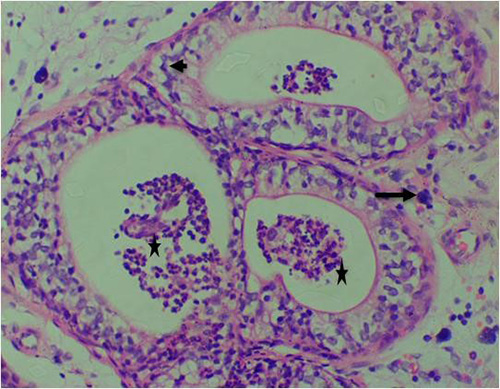
Figure 12B: Photomicrograph of group 6 rats given 3mg/kg of cadmium plus 1000mg/kg of Uvaria chamae root extract for 28days respectively showing nuclear atypia (arrow), derangement of luminal cells (short arrow) and presence of concretions with infiltrating cells in the lumen(star) (H&E) x400 magnification
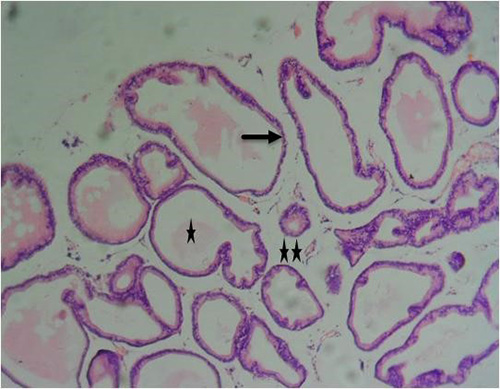
Figure 13A: Photomicrograph of group 7 rats given 150mg of casodex alone for 28days showing normal acini (arrow), well defined-lumen (star) and fibromuscular stroma (double stars), (H&E) x100 magnification
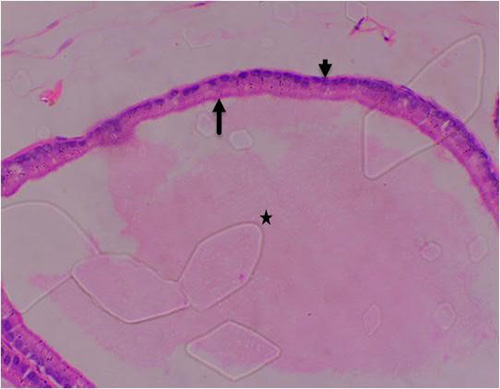
Figure 13B: Photomicrograph of group 7 rats given 150mg of casodex alone for 28days showing normal acini (arrow), well defined-lumen (star) and basal cells (short arrow), (H&E) x400 magnification
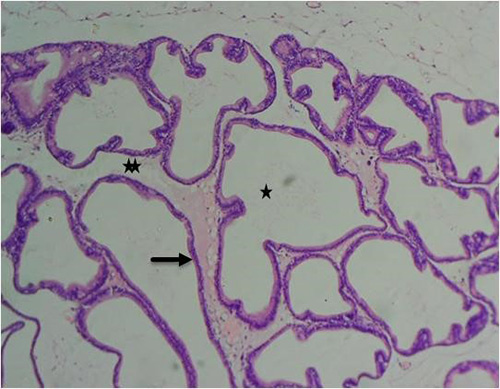
Figure 14A: Photomicrograph of group 8 rats given 2500mg/kg of Uvaria chamae root extract alone for 28days showing well defined lumen (star), normal acini (arrow) and fibromuscular stroma (double stars), (H&E)x100 magnification
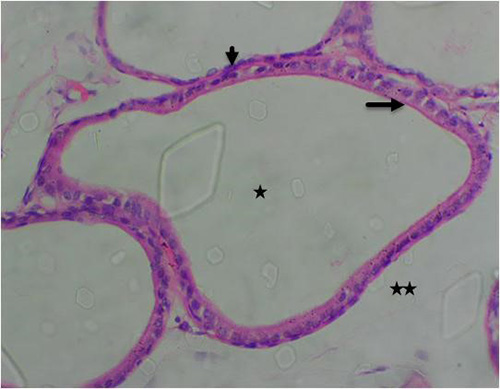
Figure 14B: Photomicrograph of group 8 rats given 2500mg/kg of Uvaria chamae root extract alone for 28days showing well defined lumen (star), normal acini with luminal cells (arrow), Basal cells (short arrow) and fibromuscular stroma (double stars), (H&E) x400 magnification
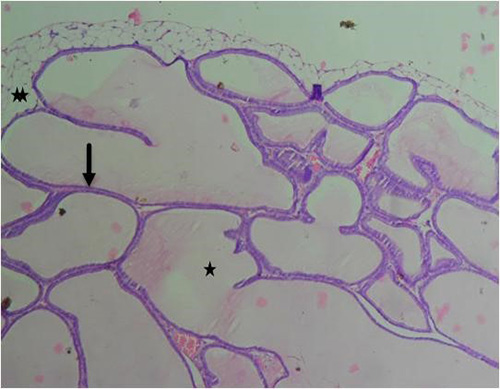
Figure 15A: Photomicrograph of group 9 rats given 1500mg/kg of Uvaria chamae root extract alone for 28days showing well-defined lumen (star), normal acini (arrow) and fibromuscular stroma (double stars), (H&E) x100 magnification
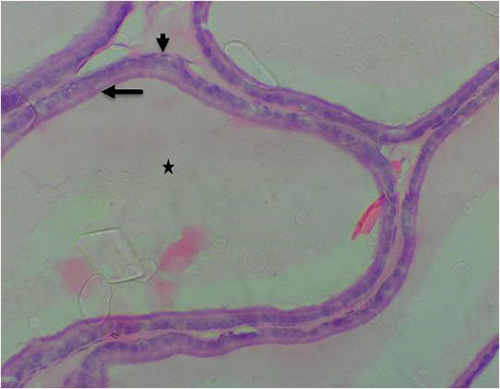
Figure 15B: Photomicrograph of group 9 rats given 1500mg/kg of Uvaria chamae root extract alone for 28days showing well-defined acini with luminal cells (arrow), basal cells (short arrow) and normal lumen (star) (H&E) x400 magnification
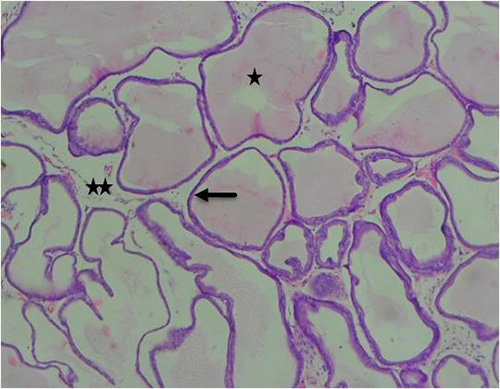
Figure 16A: Photomicrograph of group 10 rats given 1000mg/kg of Uvaria chamae root extract alone for 28days showing well defined lumen (star), normal acini (arrow) and fibromuscular stroma (star), (H&E) x100 magnification
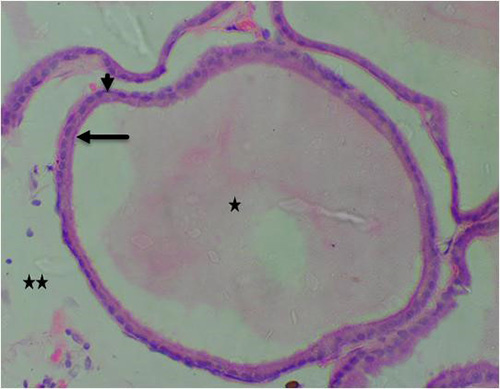
Figure 16B: Photomicrograph of group 10 rats given 1000mg/kg of Uvaria chamae root extract alone for 28days showing well defined lumen (star), normal acini (arrow) with luminal cells, Basal cells (short arrow) and fibromuscular stroma (star), (H&E) x400 magnification
Discussion
The serum levels of testosterone, LH, estradiol, FSH and prolactin were significantly increased in the cadmium alone group when compared to control and other groups (P<0.05). Testosterone is a steroid hormone having an unsaturated bond between the fourth (C-4) and the fifth (C-5) carbon atoms, a ketone group at the third carbon atoms(C-3) and hydroxyl(OH-) at the seventeen(C-17) carbon atom. It is the most important androgen secreted by the Leydig cells. Administration of cadmium may exert carcinogenic effect on the prostate of cadmium treated rats and significantly increasing the serum level of testosterone. In males, high level of testosterone can be associated with testicular tumour and prostate cancer. High level of testosterone has been reported to drive prostate cancer development and progression because prostate cancer development is promoted by androgens.18 Several studies have linked elevated testosterone level with prostate cancer. For instance, Gann et al. (1996)19 reported elevated testosterone level in about 95% of males to be associated with prostate cancer. Pierorazio et al. (2010)20 also examined the relationship between serum testosterone and free testosterone index and prostate cancer. They reported that testosterone was implicated in the progression of prostate cancer. Similarly, Yano et al. (2007)21 reported a significant increase in prostate cancer risk among men with increased testosterone level. Our study is in total agreement with the enormous amount of scientific data implicating androgens in prostate cancer pathogenesis and progression. Administration of the various doses of Uvaria chamae root extract significantly reduced testosterone levels in a dose-dependent manner when compared to control, group 2 and standard (casodex). This is because, the bio-molecules, such as polyphenols present in Uvaria chamae may act in a similar way as androgen deprivation therapy (ADT) by reducing androgen production in the testis as well as blocking the production and action of androgens throughout the animal’s body. The direct competition between polyphenols and androgens tends to be based on their structural resemblance and competition for the binding site on androgen receptors which will eventually reduce the transcriptional activity of androgen receptors on prostate cancer growth and development. These active bio-molecules in the root extract of Uvaria chamae interact with hormone receptors, cell membrane, DNA and other small molecules involved in alternative activation pathways of androgen receptors in prostate cancer such as P13K/Akt, MAPK.22-24 The pro-oxidant potentials of Uvaria chamae root extract can induce cytotoxicity in pre-cancerous and cancerous cells.25 Additionally, phenols in the plant can hinder neoplastic processes such as proliferation, metastasis and invasion.26 Cadmium significantly increased luteinizing hormone (LH) levels when compared to control and other groups. LH is a gonadotropic hormone produced and released by the anterior pituitary. LH surge was down-regulated dose-dependently by the administration of root extract of Uvaria chamae. The biomolecules present in the plant can act as a luteinizing hormone releasing hormone (LHRH) antagonist or luteinizing hormone blocker and gonadotropin releasing hormone (GnRH) antagonist. These processes control the amount of testosterone produced by the testis thereby inhibiting cancer progression. Estradiol (E2) was significantly raised in cadmium group (P<0.05) but down-regulated in other treatment groups when compared to control. Down-regulation of E2 surge can be attributed to the actions of polyphenols present in the root of Uvaria chamae; polyphenols are capable of binding to estrogen receptors which include a (ER a) and ß(ER ß) thereby antagonising or inhibiting the actions of endogenous estrogens even at lower concentrations27 and thus interfering with tumour growth and progression.28-29 Polyphenols present in Uvaria chamae has pro-oxidant properties through which they induce cell death and promote cancer cell inhibition30-31 by modulating the activity of signaling pathways involved in the proliferation of cancer cells. These pathways include mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways.28,32 Additionally, presence of polyphenols in Uvaria chamae can cause an inhibition of cyclic adenosine mono phosphate (cAMP) response element binding protein (CREB) and extracellular signal-regulated kinases 1 and 2; these processes appear to be the mechanisms that are mostly employed by polyphenols to initiate a block in the progression of cell cycle.33-34 Our investigation portrays that, the root extract of Uvaria chamae was able to modulate estrogen receptor signaling and regulate hormone related disorders. Increase in the serum level of follicle stimulating hormone (FSH) was suggestive of its role in carcinogenesis. FSH is produced in the anterior pituitary and acts on other extrapituitary organs.35 FSH acts on the seminiferous tubule to produce sperm and release follistatin, activin and inhibin.36 Multiple reports have shown that malignant and benign prostate cells produce FSH and its receptor with a possibility of increased expression in malignancy.37 Our findings corroborate the works of Crawford et al. (2014)38 who reported that FSH plays an important role in the natural history of progression of prostate cancer. Furthermore, administration of varying doses of Uvaria chamae root dose-dependently acted as GnRH antagonist on FSH levels. This is achieved by inactivating sex production enzymes which includes 5a-reductase, P450 aromatase, 17ß-hydroxysteroid dehydrogenase, tyrosine kinases and topoisomerases. It may also act by controlling the expression of genes or the activity of enzymes involved in the regulation of reproductive events.39 The anti-oxidant agents present in the plant induces the action of prostatic inhibin peptide which in turn decreases the production of FSH in the anterior pituitary. Summarily, FSH suppression by the root extract of Uvaria chamae would be analogous to testosterone suppression to inhibit the growth of prostate cancer cells. A surge in the serum level of prolactin in cadmium treated group could be associated to the hyper-prolactinemic effect of cadmium.40 Prolactin is a peptide hormone which stimulates prostate growth in laboratory animals40 and in vivo studies have reported that hyper-prolactinemia induces the proliferation and differentiation of prostate cancer.41 Prolactin has been shown to stimulate the growth of tumour in rats.41 The development of dysplasia and inflammation in rat prostate treated with estrogen was reported to be mediated by increased prolactin serum level42 whose receptors have been reported to be expressed in the prostate.43 The expression of prolactin receptors is reported to be interestingly elevated in a precursor of prostate cancer called high grade prostate intra-epithelial neoplasia (PIN).44 Technically, our result is in tandem with other research findings which suggested that increased levels of circulating prolactin induces prostate growth with a resultant appearance of prostate tumour. Surprisingly, the serum levels of prolactin were significantly reduced in Uvaria chamae treated groups (P<0.05) in a dose-dependent manner when compared to control, group 2 and standard. It is worthy to note that Uvaria chamae could be proposed to possess an anti-hyper-prolactinemic effect. Yakubu and Fayemo (2021)45 demonstrated the anti-hyperprolactinemic effect of Uvavia chamae in Wistar rats and reported a decrease in the serum levels of the hormone after administration of Uvaria chamae. A slight increase in the serum level of progesterone was noticed though significantly not different across all groups (P<0.05). Progesterone is a steroid hormone which can act as an intermediary in the steroid hormone synthesis pathway. It is well known for its role in the female reproductive organs.45 However, progesterone receptors (PGRs) have been found in prostate malignancy.46 Our findings supported that of Thea et al. (2018)47 who reported the presence of progesterone receptors (PGRA and PGRB) in both the stromal and epithelial tissues of the prostate. Yu (2013)46 also reported the role of progesterone receptors in regulating the stromal environment of the prostate. Unfortunately, the physiological functions of progesterone receptors in the normal prostate and their involvement in prostate cancer development and progression have not yet been defined.47 Generally speaking, prostate cancer is the second most leading cause of cancer deaths in men worldwide. It is worthy to note that, most lesions in the prostate are presented with complex glandular architecture.48 Interestingly, prostate cancer typically present with small gland morphology; histological evaluation of prostate cancer is required to assess the biology and progression of the disease. From our findings, the histopathological assessment of H&E stained tissue sections in cadmium alone treated rats revealed several premalignant features ranging from luminal undulation with branching, nuclear atypia, nuclear hyperchromasia, infiltration of inflammatory cells, crowded/small glands with irregular contour and rigid lumen. The glands of prostate cancer are small, crowded, and poses rigid lumen. These glands have characteristic infiltrative growth as observed in our investigation. Architecturally, glands forming prostate carcinoma are conspicuously and more crowded than normal and physically exhibit a growth pattern oriented haphazardly.48 Less differentiated prostate cancer can present with a partial or total loss of glandular structures which forms cribriform structures, fused glands and poorly delineated glands. Our findings are in tandem with the works of Moch et al. (2016)49 who reported dark amphophilic glands, nuclear crowding/stratification, irregular spacing, amphophilic cytoplasm in luminal cells and prominent nucleoli in prostate cancer. Our investigation is also in support of several reports that opined that cadmium is a potent carcinogenic substance.48 Administration of 3mg/kg of cadmium alone was able to induce premalignant features of the prostate characterized by small glands that are lined by pseudostratified tall columnar cells. Histologic changes of neoplastic epithelium of the prostate related to the administration of various doses of Uvaria chamae root extract are very distinctive which include well-defined acini without undulation and branching, normal glandular lumen and fibromuscular stroma as well as reduction of inflammatory cells. These histological changes were clearly noted in 2500mg/kg and 1500mg/kg treated animals of Uvaria chamae root extract respectively when compared to control, group 2 and standard drug (casodex). These ameliorating changes were caused by androgen deprivation ability of the root extract. The branching and undulating spaces usually observed in prostate cancer was described by Tetu et al. (1991)50 as a hemangiopericytoma-like arrangement. The root extract of the plant dose-dependently ameliorated the cellular infiltrative pattern of ribbons and nests of cells and even cells of inflammatory origins. Dhom and Degro (1982)51 predicted a similar relationship between the degree of tumour growth after androgen ablation and the clinical response of cancer cell proliferation. Amelioration of these precancerous/cancerous features by the root extract of Uvaria chamae could also be linked to its ability to decrease the blood circulationg levels of steroid hormones as witnessed in this investigation, since the surge of these hormones drive prostate cancer growth and development. Inhibiting action of the plant on steroid hormones to inhibit cellular functions and growth is thought to be at a level of processes involved with RNA and protein synthesis.52 Furthermore, these inhibiting actions of Uvaria chamae root could be attributed to its Nrf2 inducing ability. Nrf2 is a regulatory transcription factor responsible for inducing genes associated with xenobiotic and oxidative stresses.53 The significance of Nrf2 inducers was reported to be up-regulated by phenolic compounds which could prevent chemical carcinogenesis.54 Thus, the root extract of Uvaria chamae can be considered a potent anti-cancer and anti-inflammatory agent.15,55 Godwin et al. (2023)56 in their studies reported the cytotoxic activity of Uvaria chamae root extract on cancer cell lines with a reported increase in pro-apoptotic BAX and a decreased expression of Bcl-2 across four cancer cell lines such as A549, MCF-7, RD and HeLa. This current study is in support of the study by Temidayo et al. (2021)57 who reported the anti-cancer capacity of Uvaria chamae due to its potent quality as a good Nrf2 inducer. Anti-oxidants such as phenol, flavonoids, alkaloid in Uvaria chamae support its anti-cancer property.58 The anti-carcinogenic property of phenols is based on their ability to initiate apoptosis and the regulation of copper ion and chromatin assemblage.59 The polyphenols in the plant is believed to interact with cancer cell proteins and thereafter inhibit the growth of these cells. Polyphenols directly initiate a chemical bonding with the cancer proteins and disrupt their cancer promoting activities through the following processes; methylation, phosphorylation and acetylation.60 Similarly flavonoid present in the plant exhibit high free scavenging potentials by initiating apoptosis through the extrinsic and intrinsic signaling pathways and the destruction of cancer cell mitochondria.61 Flavonoids hinder the expression of NF-kB expression, a protein needed for proliferation and the survival of tumour cells.62 Alkaloids present in the root extract of Uvaria chamae also disrupts prostate cancer progression and cancer cell growth63 by initiating programmed cell death and stimulating the end of cancer cell cycle mostly at the G1-phase or G2 or M-phase.64 From the current study, the root extract dose-dependently ameliorated tumour cell infiltration and it is a well known fact that, alkaloids are potent tumour progression inhibitors.65-66 Saponin present in the plant may have contributed in its ameliorating prostate cancer growth and progression by exhibiting immunomodulatory responses through the interplay of cytokines.64 They are reported to repress cancer cells by activating apoptosis and terminating the cancer cell cycle at G2/M-phase.67 Moreover, the anti-cancer property of root extract could also be linked to the presence of triterpenes as revealed from phytochemical screening.58 Triterpenes are believed to initiate cancer cell apoptosis by stimulating the signaling pathway of the death receptor (DR-5).68 Triterpenes also cause apoptosis through the processes of respectively up-regulating and down-regulating pro-apoptotic and anti-apoptotic proteins69; they can also hinder the migration of tumour cells through the process of mitigating MMP-2 and MMp-9 expression, hindering the secretion of BFGF/VEGF-A (angiogenic factors) and vascular endothelial growth factor inhibition.70 Tannin in the plant causes S-phase cell cycle obstruction, up-regulation of cyclin E, down-regulation of cyclins A and B1, caspase-3 and caspase-9 induction and initiation of mitochondrial secretion of cytochrome C.71 Quinones present in the plant activate apoptosis of cancer cell via the process of stimulating the Fas pathway, p53 pathway and c-junN-terminal kinase.72 It causes inhibition of cancer cell proliferation by hindering GI, G2/M and S-Phases of cell cycle.73 However, there were no observable histopathological changes in the tissue sections of Uvaria chamae alone treated animals (2500mg/kg, 1500mg/kg and 1000mg/kg) respectively when compared to control. This is borne out of the fact that, the root extract of the plant is relatively safe as revealed in the toxicity study conducted by Edem et al., 2023.58
Conclusion
This study has shown that cadmium possesses carcinogenic potentials in the prostate of adult male Wistar rats. This study also demonstrates that Uvaria chamae root extract has scavenging property and ameliorative potentials against cadmium induced carcinogenic potentials in the prostate of Wistar rats. This ameliorative tendency was exhibited in its ability to decrease serum levels of testosterone, estrogen, LH, FSH and prolactin. Further ameliorative activity of the root was observed in the histology of the prostate gland after treatment with cadmium. These findings provide a rationale for the usefulness of Uvaria chamae root as an adjunct treatment option in the management of premalignant or cancerous conditions of the prostate in Nigeria, because Uvaria chamae root possesses strong anti-cancer tendencies; further research should be conducted to isolate these bioactive ingredients responsible for this important therapeutic ability for the sole purpose of anti-cancer drug production.
Acknowledgement
Special thanks to TETFUND (Tertiary Education Trust Fund) for supporting and sponsoring this research.
References
- Calcaterra, I, Iannuzzo G, Dell'Aquila F, Di-Minno MND. Pathophysiological role of synovitis in hemophilic arthropathy development: a two-hit hypothesis. Fronts. 2020; 11:541.
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epi Bio Prev. 2010; 19(8): 1893-907.
- Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019; 9(4): 217-222.
- Kimura T. East meet west: Ethnic differences in prostate cancer epidemiology between east asians and caucasians. Chi J Cancer. 2012; 31(9): 421-429.
- Bleyer A, Spreafico F, Barr R. Causation of increased prostate cancer in young men. Oncosci. 2021; 8:37-38.
- Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer Statistics for african american /black people. Cancer J Clin. 2002; 72(3): 202-229.
- Castillejos-Molina RA, Gabilondo-Navarro, FB. Prostate cancer. Salud pub de Mex. 2016; 58(2): 279-284.
- Costello LC, Franklin RB. A comprehensive review of the role of zinc in normal prostate function and metabolism and its implications in prostate cancer. Arch Biochem Biophysics. 2016; 1 (611):100-112.
- Gennigens C, Caux CM, Droz JP. Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncolol Hematol. 2006; 58:124–145.
- Nock NL, Liu X, Cicek MS. Polymorphisms in polycyclic aromatic hydrocarbon metabolism and conjugation genes, interactions with smoking and prostate cancer risk. Cancer Epi Biom Prev. 2006; 15(4):756–61.
- Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010; 100(4): 693-701.
- Kjellstrom T, Friberg L, Rahnster B. Mortality and cancer morbidity among cadmium-exposed workers. Environ Health Perspect. 1979; 28:199–204.
- Nordberg GF, Goyer RA, Clarkson TW. Impact of effects of acid precipitation on Toxicity of metals. Environ Health Perspect. 1985; 63:169–180.
- Matovic V, Buha A, Ðuki´c-Cosic D, Bulat Z. Insight into the oxidative stress induced by lead and /or cadmium in blood, liver and kidneys. Food Chem Toxicol. 2015; 78, 130–140.
- Edem GD, David JU, Mbadugha CC, Ejeguo A, Friday DG. Cadmium toxicity in the seminal vesicles and lipid profile of Wistar rats: Can Uvaria chamae ameliorate the effect?. Clin Res Clin Rep. 2024; 3(1): 1-8.
- Bongers F, Parren MP, Traore D. Forest climbing plants of west africa: diversity, ecology and management. CABI Publishing international,Wallingfork, UK. 2005; 273.
- Iwu MM. Handbook of African Medicinal Plants. 2nd Edition, CRC group, London. 2014; 125.
- Jason EM, Kevin LB, Alan WP. Testosterone and prostate cancer: an evidence- based review of pathogenesis and oncologic risk. Ther AdvUrol. 2015; 7(6): 378-387.
- Gann P, Hennekens C, Ma J, Longcope C, Stampfer M. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer inst. 1996; 88: 1118-1126.
- Pierorazio P, Ferrucci L, Kettermann A, Longo D, Metter E, Carter H. Serum testosterone is associated with aggressive prostate cancer in older men: Results from the Baltimore Longitudinal Study of aging. BJU Intl. 2010; 105: 824-829.
- Yano M, Imamoto T, Suzuki H, Fukasawa S, Kojima S, Komiya A. The clinical potential of pretreatment serum testosterone level to improve the efficiency of prostate cancer screening. Rob Urol. 2007; 51: 375-380.
- Basak S, Pookot D, Noonan EJ, Dahiya R. Genistein down regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol Cancer Ther. 2008; 7: 3195-3202.
- Jones SB, Deprimo SE, Whitfield ML, Brooks JD. Resveratrol- induced gene expression profiles in human prostate cancer cells, cancer epidemiology. Biom Prev. 2005; 14: 596-604.
- Liu KC, Yen CY, Wu RS, Yang JS, Lu HF, Lu KW, Lo C, Chen HY, Tang NY, Wu CC. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ Toxicol. 2014; 29: 428-439.
- Gao X, Schotter B. Reduction-oxidation pathways involved in cancer development: A systematic review of literature review. Oncotarget. 2017; 8: 51888-51906.
- Li Y, Che M, Bhagat S, Ellis KL, Kucuk O, Doerge DR, Abrams J, Cher ML, Sarkar FH. Regulation of gene expression and inhibition of experimental prostate cancer, bone metastasis by dietary genistein. Neopla. 2004; 6: 354-363.
- Manuella C, Virginia SF, Emiliano MM, Marco F. Beyond the antioxidant activity of dietary polyphenols in cancer: the modulation of estrogen receptors signaling. Intl J Mol Sci. 2018; 19(9): 2624.
- Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP. Polyphenols and human health: prevention of disease and mechanisms of action. Nutr. 2010; 2: 1106-1131.
- Russo GL, Tedesco I, Spagnuolo C, Russo M. Antioxidant polyphenols in cancer treatment: Friend, foe or foil. Sem Cancer Biol. 2017; 46: 1-13.
- Scalbert, A, Manach C, Morand C, Remesy C, Jimenez L. Polyphenols and prevention of diseases. Crit Rev Food Sci Nutr. 2005; 45: 287-306.
- Sergediene E, Jonsson K, Szymusiak H, Tyrakowsa B, Rietjens IM, Cenas N. Pro-oxidant toxicity of polyphenolic antioxidants to hi-60 cell: description of quantitative structure-activity relationships. FEBBS lett. 1999; 462: 392-396.
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signaling pathways. Mol Nutr Food Res. 2008; 52: 507-526.
- Corona G, Deiana M, Incani A, Vauzour D, Dessi MA, Spencer JP. Inhibition of P38/creb phosphorylation and cox-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem Biophy Res Comm. 2007; 362: 606-611.
- Guihard C, Pedruzzi E, Fay M, Marie JC, Braut-Boucher F, Daniel F, Grodet A, Chastre E. Dihydroxyphenylethanol induces apoptosis by activating serine/threonine protein phosphatase pp2a and promotes the endoplasmic reticulum stress response in human colon carcinomacells. Carcinogenesis. 2006; 27(18): 1812-1827.
- Garde SV, Sheth AR, Shah MG. An extrapituitary source of follicle stimulating hormone: Occurrence, Localization and de noco biosynthesis and its prostatic specific antigen prostatic specific acid phosphatase, and prostatic inhibin peptide. Prostate. 1991; 18(4): 271-287.
- David C, Andrew S. The Role of FSH and LH in prostate cancer and cardiometabolic comorbidities. Can J Urol. 2020; 27(2): 10167-10173.
- Mariani S, Salvatori L, Basciani S. Expression and Cellular Localization of FSH receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer, J Urol. 2006; 175(6): 2072-2077.
- Crawford ED, Rove KO, Schally AV. The role of the FSH system in the development and progression of prostate cancer. Am J Hematol Oncol. 2014; 10(6): 5-13.
- Yousif AN. Effect of flax seed on some hormonal profile and genomic DNA concentration in karadi lambs. IOP conference Series: Earth Environ Sci. 2019; 388: 17-18.
- Par S, Sabina R, Ulf-Hakan S, Elio R, Goran H, Anders B, Rudolf K. Plasma prolactin and prostate cancer risk: a propective study. Intl J Cancer. 2001; 92: 463-465.
- Johnson MP, Thompson SA, Lubroff DM. Differential effects of prolactin on rat dorsolateral prostate and R32 and prostatic tumour sublines. J Urol. 1985; 133: 1112-1120.
- Lane KE, Leav I, Ziar J, Bridges RS, Rand, WM, Ho SM. Suppression of testosterone and estradiol-17beta-induced dysplasia in the dorsolateral prostate of Noble rats by bromocriptine. Carcinogenesis. 1997; 18:1505–1510.
- Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest.1997; 99:618–627.
- Leav I, Merk FB, Lee KF, Loda M, Mandoki M, McNeal JE. Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am J Pathol. 1999; 154:863–8670.
- Gardner DG. Greenspan’s basic and clinical endocrinology, McGraw Hill companies, inc. 2011; 213-215.
- Yu Y. Expression and function of the progesterone receptor in humane prostate stroma provide novel insights to cell proliferation control. J Clin Endocrinol Metab. 2013; 98: 2887-2896.
- Thea G, Elin R, Sigve A, Kaja S, Mehrdad R, Tom D, Nora Yngve N, Roy M, Samer A, Lill-Tove B. Progesterone receptors in prostate cancer: Progesterone receptor B is the isoform associated with disease progression. Sci Rep. 2018; 8: 11358.
- Zhou M. High-grade prostatic intraepithelial neoplasia, PIN-like carcinoma, ductal carcinoma, and intraductal carcinoma of the prostate. Modern Pathol. 2018; 31: s71-s79.
- Moch H, Humphrey PA, Ulbrigh TM, Reutere VE. WHO classification of tumours of the urinary system and male genital organs. International Agency for Research on Cancer: Lyon, France, 2016; 1-6
- Tetu B, Sringley JR, Boivin JC, Dupont A, Monfette G, Pinault S, Labrie F. Effect of combination endocrine therapy on normal prostate and prostatic adenocarcinoma. a histopatholic and immunohistochemical study. Am J Surg Pathol. 1991; 15, 11-120.
- Dhom G, Degro S. Therapy of prostatic cancer and histopathologic follow-up. Prostate.1982; 3: 531-542.
- David AK. Karnofsky’s early contributions to cancer research helped establish oncology as a medical discipline. Cancer Res.1970; 30: 549-550.
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res comm. 1997; 236(2): 313-322.
- Hayes J. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001; 174(2):103-113.
- Edem GD, David JU, Okon KA, Thompson HI. Relationship between Cadmium Toxicity, Kidney Function Disturbances and Urinary Bladder Inflammation: The Role of Uvaria chamae in Mitigating these Effects. Drug Discov. 2024; 18e6dd1968.
- Godwin EA, Toluwanimi EA, Uwem EG, Bob IM, Olubusuyi MA, Johnson AA, Omonike OO, Patrick E. Cytotoxic action of the leaves of Uvaria chamae and Dicliptera paniculata from Nigeria mediated through intrinsic apoptotic pathway induction in four cancer cell lines. Phytomed. 2023; 3(2): 100423.
- Temidayo DP, Stephanie TG, Kenneth JR, Olufunsho A, Nicola MD, Oluyemi A, Satyajit DS. Potent Nrf2 inducing, antioxidant and anti-inflammatory effects and Identification of Constituents Validate the anti-cancer use of Uvaria chamae and Olax subscorpioidea, BMC Compl Med Ther. 2021; 21: 234.
- Edem GD, Sakpa CL, Ezeuko VC. Exploring the Scientific Basis behind the Therapeutic Efficacy of Uvaria chamae: A Major Plus to Alternative Medicine. J New Med Innov Res. 2023; 4(6): 1-7
- Azmi AS, Bhat SH, Hanif S, Hadi SM. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties. FEBS Lett. 2006; 580(2):533–538.
- Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys.2014; 559:91–99.
- Kumar S, Pathania AS, Saxena AK, Vishwakarma RA, Ali A, Bhunshan S. The anticancer potential of flavonoids isolated from the stem-bark of Erythrina suberosa through induction of apoptosis and inhibition of STAT signaling pathway in human leukaemia HL-60 cells. Chemico-Biological Interactions. 2013; 205(2):128–137.
- Greenwell M, Rahman PKSM. Medicinal plants: their use in anticancer treatment. Intl J Pharm Sci.2015; 6(10):4103–4112.
- Diogo CV, Machado NG, Barbosa IA, Serafim TL, Burgeiro A, Oliveira PJ. Berberine as a promising safe anti-cancer agent – is there a role for mitochondria? Curr Drug Targ. 2011; 12(6):850–859.
- Sun Y, Xun K, Wang Y, Chen X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs. 2009; 20(9):757–769.
- Tang F, Wang D, Duan C. Berberine inhibits metastasis of nasopharyngeal carcinoma 5-8F cells by targeting rhokinase-mediated ezrin phosphorylation at threonine 567. J Biol Chem. 2009; 284(40):27456–27466.
- Debiton E, Madelmont JC, Legault J, Barthomeuf C. Sanguinarine induced apoptosis is associated with a nearly and severe cellular glutathione depletion. Cancer Chemo Pharmacol. 2003; 51(6):474–482.
- Man S, Gao W, Zhang Y, Huang L, Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia. 2010; 81(7):703–714.
- Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005; 12(6):657–666.
- Amico V, Barresi V, Condorelli D, Spatafora C, Tringali C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): identification and structure-activity relationships. J Agric Food Chem. 2006; 54(3):810–814.
- Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007; 28(6):1347–1355.
- Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma CaCo-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006; 17(9):611–625.
- Yeh FT, Wu CH, Lee HZ. Signaling pathway for aloe-emodin-induced apoptosis in human H460 lung non-small carcinoma cell. International Journal of Cancer. 2003; 106(1):26–33.
- Kuo PL, Lin TC, Lin CC. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci. 2002; 71(16):1879–1892.