Anaemia of inflammation in sickle cell disease: A review of aetiopathogenesis, management, and prevention of sterile and septic inflammation in patients with sickle cell disease
Ahmed SG1, Ibrahim UA2
Abstract
Background: SCD is strongly associated with sterile inflammation (STERIN) and septic inflammation (SEPTIN). Thus, in addition to haemolysis, anaemia in SCD has dual ‘inflammatory’ components; (1)-anaemia of sterile inflammation (ASTERIN) and (2)-anaemia of septic inflammation (ASEPTIN). Hence, the need to explore the relatively understated but important concept of ‘anaemia of inflammation in SCD’.
Objectives: The objectives of this review are tri-fold; (1)-To reappraise the aetiopathogenesis of STERIN and SEPTIN in SCD; (2)-To highlight the roles of STERIN and SEPTIN in causation of ASTERIN and ASEPTIN in SCD; and (3)-To underscore the roles of mitigators of STERIN and SEPTIN in managing/preventing ASTERIN and ASEPTIN in SCD.
Methodology: Literature search was done using terms relevant to ‘SCD/inflammation/anaemia’. Only articles on aetiopathogenesis, management, and/or prevention of STERIN/ASTERIN and/or SEPTIN/ASEPTIN were selected.
Results: Hyper-inflammation in SCD has dual components; STERIN (caused by tissue injury/haemolysis) and SEPTIN (caused by infections). Accordingly, ‘anaemia of inflammation in SCD’ has dual components; ASTERIN and ASEPTIN. ASTERIN is driven by STERIN, which is relentless and occurs even in steady-state, and is often aggravated by crisis and/or autoimmune diseases. ASEPTIN is driven by SEPTIN, which occurs only during infections. Hence, during infection, ASTERIN and ASEPTIN act synergistically to worsen anaemia and increase transfusion risk in SCD. Mitigators of STERIN (e.g., hydroxyurea, immune-modulators) and SEPTIN (e.g., anti-microbials, vaccines) have beneficial roles in managing and preventing ASTERIN and ASEPTIN in SCD.
Conclusion: Anaemia in SCD has significant inflammatory components. Hence, managing/preventing STERIN and SEPTIN are important strategies for down-regulating ASTERIN and ASEPTIN, improving Hb-concentration, and reducing transfusion risk in SCD.
Key Words: Sickle Cell Disease, Anaemia of Inflammation, Sterile Inflammation, Septic Inflammation, Aetiopathogenesis, Management, Prevention.
Introduction
The sickle cell haemoglobin (HbS) is a variant of the normal haemoglobin (HbA). HbS arises as a result of GAG>GTG base transition at codon-6 of the ß-globin gene on chromosome-11, which corresponds to a replacement of glutamic acid (a polar amino acid) by valine (a neutral amino acid) in the sixth position of the ß-globin chain (ßGlu6Val).1,2 As a result of this substitution, HbS has less anionic potential, slower electrophoretic mobility, and reduced deoxygenated solubility that leads to polymerization and red cell sickling.1,2 The prevalence of sickle ß-gene in tropical Africa is as high as 25-30%.3 The prevalence is high because the sickle cell trait (SCT) protects against severe malaria3 and confers survival advantage through natural selection,4 balanced polymorphism,5 as well as immunological and biochemical protective mechanisms against the parasite.6 There are at least five different sickle ß-gene mutation haplotypes that vary in HbF levels and disease severity. The Arab-Asian and Senegal haplotypes are associated with relatively higher HbF levels and milder sickle cell disease (SCD), while the Benin, Bantu, and Cameroon haplotypes are associated with relatively lower HbF levels and severer SCD.7
The red cells of persons with SCT have the HbAS dual phenotype, thus containing both HbS (20-40%) and HbA (60-80%).8 The relative preponderance of HbA in SCT red cells prevents sickling and undue haemolysis under physiological circumstances.8 Consequently, SCT red cells have a normal life span, and persons with SCT have normal life expectancy.9 HbS gene is thus genetically recessive, and SCT carriers are essentially asymptomatic except for the occasional occurrence of renal papillary necrosis,8 splenic infarction at high altitude,10 or bone pain upon exposure to certain haematopoietic growth factors.11 SCD arises from the homozygous inheritance of HbS gene or double heterozygosity of HbS gene with another haemoglobinopathy gene (e.g., HbSC, HbSD, HbSE, HbSO, HbSßthal).1 Red cell sickling, microvascular occlusion, infarctions, painful vaso-occlusive crisis (VOC), and haemolysis are pathognomonic of SCD.2 Consequently, the pathophysiology of SCD is dominated by pain (due to infarction-induced tissue necrosis), anaemia (due to haemolysis), recurrent infections (due to infarctive autosplenectomy and immune dysfunctions), and inflammation (due to tissue injury, haemolysis, and infections).12-16
Hyper-inflammation in SCD has two components, viz: ‘sterile inflammation’ (STERIN) (due to tissue injury and haemolysis) and ‘septic inflammation’ (SEPTIN) (due to infections).17 The pathophysiology of SCD is thus characterized by a hyper-inflammatory state with high levels of pro-inflammatory cytokines (e.g., TNF-a, IFN-?, IL-1ß, IL-6, and IL-8).17,18 Unfortunately, SCD patients are usually unable to generate sufficient amount of anti-inflammatory cytokines (e.g., TGF-ß, IL-4, IL-10) to counter-balance the deleterious effects of pro-inflammatory hyper-cytokinemia.19 Consequently, the imbalance between pro- and anti-inflammatory cytokines is often skewed in favour of pro-inflammatory cytokines,19 and the imbalance is further accentuated during VOC.20 The resultant pro-inflammatory hyper-cytokinemia causes chronic inflammation, which is undesirable in SCD because it increases the risks of oxidative stress, VOC, stroke, acute chest syndrome, pulmonary hypertension, priapism, leg ulcer, nephropathy, and multi-organ failure syndromes14; neuro-cognitive dysfunction21; and accelerated premature ageing at genetic,22 epigenetic,23,24 and organismal25 levels. Moreover, hyper-inflammation in SCD will also undesirably aggravate anaemia by suppressing renal production of erythropoietin (EPO) and reducing the responsiveness of erythroid precursors to EPO,26 thus decreasing red cell production in keeping with the pathophysiology of anaemia of inflammation.27 We therefore reckon that, in addition to haemolysis, anaemia in SCD has a significant ‘inflammatory’ aetiology due to STERIN and SEPTIN, each of which is capable of inducing relative EPO deficiency,26 leading to anaemia of sterile inflammation (ASTERIN) and anaemia of septic inflammation (ASEPTIN) respectively.27 The aforementioned pathophysiologic scenario underscores a relatively understated but pathophysiologically important concept of the existence of ‘anaemia of inflammation in SCD’, which has two separate but inter-related components, viz: ASTERIN and ASEPTIN. There is therefore the need to understand the pattern and role of inflammation in the causation of anaemia in SCD. The clinico-pathological perspectives of inflammation vis-à-vis anaemia in SCD are fragmented in the literature. Hence, the aim of this overview is to present a comprehensive but concise narrative review with triple objectives: (1) to reappraise the aetiopathogenesis of STERIN and SEPTIN in SCD; (2) to highlight pathophysiologic roles of STERIN and SEPTIN on erythropoietin production and function in the causation of ASTERIN and ASEPTIN in SCD; and (3) to underscore the beneficial roles of mitigators of STERIN and SEPTIN in the management and prevention of ASTERIN and ASEPTIN in SCD.
Methodology: Literature search and selection
Literature search was conducted using relevant search terms: ‘sickle cell disease, anaemia, pathophysiology, sterile inflammation, tissue necrosis, reperfusion, haemolysis, iron overload, haemostasis activation, complement activation, septic inflammation, acute infections, chronic infections, autoimmune disorders, connective tissue diseases, chronic inflammation, anti-inflammatory cytokines, pro-inflammatory cytokines, erythropoietin suppression, anaemia of inflammation’ in various combinations in Pub Med, Medline, Bing, Google Scholar, and other online search engines. Only articles that reported on aetiology, pathogenesis, management, and/or prevention of STERIN, ASTERIN, SEPTIN, and/or ASEPTIN in patients with SCD were selected for this review. The search was unrestricted by year or place of publication, and the years of selected publications ranged from 1970 to 2024. A total of 149 relevant publications were selected, which included 117 peer-reviewed full articles, 29 peer-reviewed case series and reports, 2 edited online textbooks, and 1W.H.O. news bulletin as listed in the reference section.
Results
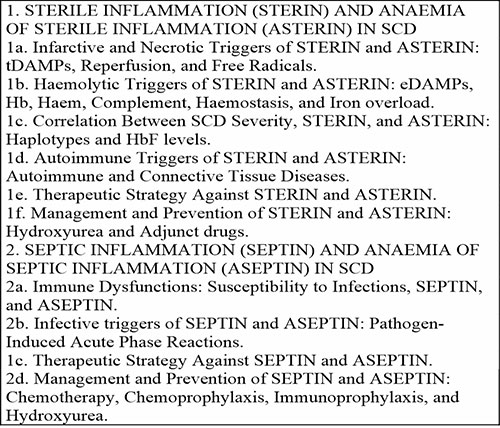
The literature revealed that hyper-inflammation in SCD falls into two main aetiopathogenetic categories; STERIN and SEPTIN. STERIN is triggered and sustained by two factors; tissue injury (due to vaso-occlusive necrosis and/or comorbid autoimmune connective tissue diseases) and haemolysis. The severity of STERIN is correlated with SCD severity vis-à-vis variations in haplotypes and HbF levels. SEPTIN is driven by immune dysfunction and susceptibility to infections, which trigger pathogen-mediated inflammatory responses. Both STERIN and SEPTIN would undermine erythropoietin production/function, suppress compensatory erythropoiesis, and lead to the development of ASTERIN and ASEPTIN, which would ultimately aggravate the background haemolytic anaemia in SCD. The classification, aetiopathogenesis, management, and prevention of STERIN and SEPTIN vis-à-vis ASTERIN and ASEPTIN in SCD are outlined in Table 1, and subsequently discussed vis-à-vis anaemia of inflammation in SCD as elucidated in the discussion section.
Table 1: Classification, aetiopathogenesis, management, and prevention of inflammation and anaemia of inflammation in SCD
Discussion
Anaemia of inflammation in SCD is caused by STERIN and SEPTIN, both of which undermine erythropoietin production, diminish compensatory erythropoiesis, and suppress optimal red cell production, ultimately resulting in ASTERIN and ASEPTIN. Both ASTERIN and ASEPTIN aggravate anaemia and increase the risk of red cell transfusion in SCD.
1. STERIN and ASTERIN in SCD: Aetiology and Pathogenesis
Undoubtedly, red cell sickling and tissue infarction are more prominent during VOC.12 Nonetheless, even in steady state, SCD is characterized by subclinical but incessant red cell sickling,28 which leads to tissue infarction, haemolysis, and STERIN.29 The role of steady state STERIN and its suppressive effect on EPO production is buttressed by earlier findings of high levels of inflammatory markers29,30,31 coupled with inappropriately low levels of EPO among SCD patients even in steady state in the absence of infection or renal dysfunction.32,33 The aforementioned combination of STERIN29,30,31 and inappropriately low level of EPO32,33 in steady state is consistent with the pathophysiology and existence of ASTERIN in patients with SCD.27,29 Steady state tissue infarction and haemolysis are two intertwined triggers of STERIN,29 which down-regulates EPO production and function,26,27 and lead to ASTERIN in SCD. On the one hand, most pro-inflammatory cytokines (notably IL-1 and IL-6) can ‘directly’ diminish the production of EPO and decrease responsiveness of erythroid EPO receptors.26,27 On the other hand, some pro-inflammatory cytokines (notably IFN-a) have been shown to also ‘indirectly’ down regulate EPO receptor responsiveness via their interactions with haemolysis-derived haem as recently demonstrated in SCD models.34 We thus surmise that the tripartite effects of hyper-inflammation due to STERIN,17,18,29-31,35 inappropriately low levels of EPO,32,33 and diminished EPO receptor responsiveness,34 create ASTERIN, which undesirably depresses erythropoietic response to the chronic haemolysis in SCD. The literature reveals three main pathophysiologic causes of STERIN and ASTERIN in SCD; viz: tissue infarction, haemolysis, and autoimmune and connective tissue diseases. The pathophysiologic roles of tissue infarction, haemolysis, and autoimmune and connective tissue diseases in the causation of STERIN and ASTERIN in SCD is elucidated in subsequent paragraphs.
1a. Infarctive and Necrotic Triggers of STERIN and ASTERIN in SCD: tDAMPs, Reperfusion, and Free Radicals
Vaso-occlusion leads to tissue injury and necrosis, which is usually followed by reperfusion.29,36 Repeated episodes of vaso-occlusion and reperfusion contribute to ischemia-reperfusion injury.29,36 Ischemia-reperfusion injury leads to generation of reactive oxygen species (ROS), microvascular dysfunction, activation of innate and adaptive immune responses, and eventually cell death.29,36 ROS-dependent damage of cellular proteins, lipids, DNA, and RNA contribute to activation of cell death via apoptosis, necrosis, autophagy, and release of neutrophil extra-cellular traps (NETs), all of which eventually lead to the release of various tissue-derived damage associated molecular patterns (tDAMPs).29,36,37 In addition to promotion of toll-like receptor-4 (TLR4) activity in the plasma of SCD patients, tDAMPs promote innate immune response by priming TLR4 signaling in endothelial cells and leukocytes.29,36,37 Consequently, TLR4 signaling causes activation of nuclear factor kappa B (NF-?B), mitogen-activated-protein-kinase, and type-I interferon pathways, all of which result in the production of wide ranging pro-inflammatory cytokines and chemokines (e.g., TNF-a, IFN-?, IL-1ß, IL-6, and IL-8) that contribute to the development of STERIN and ASTERIN in SCD.29,36,37 From pathophysiologic perspective, VOC would undesirably cause acute aggravation of pre-existing steady state infarcts28 and worsen steady state STERIN and ASTERIN.30,31,35 This is consistent with previous reports that VOC was associated with the development of additional pro-inflammatory changes, which included elevation in the levels of pro-inflammatory cytokines and chemokines such as CD40L, IL-6, IL-8, and IL-18 as well as rise in acute phase reactants such as C-reactive protein, Pentraxin-3, and Leukotriene-B4.35,38
1b. Haemolytic Triggers of STERIN and ASTERIN in SCD: eDAMPs, Hb, Haem, Complement, Haemostasis, and Iron Overload
Circulating irreversibly sickled red cells as well as sickled and dense red cells that are trapped in the microvasculature and necrotic tissues invariably undergo haemolysis,16 which is an important factor in the continuation and propagation of STERIN in SCD.29,35 About two thirds of steady state haemolysis in SCD is extravascular and is attributable to the interactions between sickled red cells and the reticuloendothelial system.16 The remaining one third of steady state haemolysis in SCD is intravascular and is attributable to cytolytic effects of free radicals and/or increased fragility of irreversibly sickled red cells.16 Like other haemolytic diseases, SCD is associated with elevated levels of xanthine oxidase.16 Xanthine oxidase catalyzes the oxidation of hypoxanthine to uric acid; a reaction that generates cytolytic free radicals that injure red cell membranes and serves as an important driver of steady state intravascular haemolysis in patients with SCD.16 Intravascular haemolysis in SCD leads to the production of cell-free Hb, haem, and erythrocyte-derived DAMPs (eDAMPs).13,29,35,39 Upon release from haemolysed sickled cells, eDAMPs would act synergistically with various tDAMPs to escalate the level of STERIN and ASTERIN in SCD.13,29,34-37,39 Furthermore, redox reactions in the plasma lead to an elevated production of haem and its oxidized form, haematin.13,29,34,35,39 Both haem and haematin are potent TLR4 agonists that contribute to a pro-inflammatory and procoagulant state, which is characterized by activated leukocytes, platelets, endothelial cells, tissue factor, nitric oxide depletion, and generation of ROS with resultant high level of oxidative stress leading to STERIN and ASTERIN.31,35,36,39,40 In addition, membrane surfaces of sickled red cells and red cell fragments of haemolysed red cells serve as template for excessive aseptic activation of complement and haemostasis.41,42 Activated components of complement and haemostatic pathways invariably trigger inflammatory reactions in patients with SCD.41,42 Moreover, chronic sickle cell haemolysis often leads to recurrent transfusion and iron overload,43 which is associated with excessive generation of free radicals and development of hyper-inflammatory state in patients with SCD.44 For the aforementioned reasons, any form of hyperhaemolytic crisis would invariably aggravate pre-existing steady state haemolysis16 and worsen steady state STERIN and ASTERIN30,31,35 by releasing more eDAMPs, Hb, haem, and red cell membrane fragments into circulation.13,45
1c. Correlation Between SCD Severity, STERIN, and ASTERIN: Effects of Haplotypes and HbF Levels
Previous studies had revealed pathophysiologic correlation between SCD severity and level of steady state STERIN. For example, SCD beta S-globin haplotypes that produce lower level of HbF (e.g., Bantu haplotype) are characterized by frequent infarctions and higher haemolysis,46 which resulted in higher levels of steady state STERIN as previously reported.47 In contradistinction, beta S-globin haplotypes that produce higher level of HbF (e.g., Benin haplotype) are characterized by less severe SCD with infrequent infarctions and lower haemolysis,46 which resulted in lower levels of steady state STERIN.47 The aforementioned ‘haplotype-inflammation’ correlation study47 underscored the protective role of HbF against frequent infarction and severe haemolysis, leading to less severe steady state STERIN in SCD patients with high HbF producing haplotypes.46 Consequently, high HbF is associated with less severe ASTERIN (resulting in modest steady state anaemia) and less severe clinical manifestations of other inflammation-associated complications (including multi-organ dysfunctions) as already reported in SCD patients with high HbF producing haplotypes.46 The aforementioned haplotype-inflammation correlation study47 also underscored the therapeutically rational and efficacious role of epigenetic amplifiers of HbF (notably hydroxyurea) in the management and prevention of STERIN and ASTERIN in SCD.
1d. Autoimmune Triggers of STERIN and ASTERIN in SCD: Autoimmune and Connective Tissue Diseases
Splenectomy (whether surgical or due to autosplenectomy) constitutes a significant risk factor for not only overwhelming sepsis, but also for triggering autoimmune diseases (AIDs) as depicted in both humans and experimental animals.48-52 The natural course of SCD is characterized by autosplenectomy due to repeated episodes of vaso-occlusive infarctions that shrink the spleen into a small functionless siderofibrotic nodule.53-55 Therefore, asplenia due to autosplenectomy constitutes a risk factor for AIDs in patients with SCD.56 This is consistent with previous studies, which had shown that patients with SCD often produce high titre autoantibodies against a wide range of auto-antigens expressed in solid organs,57,58 and more recent studies have reported significantly higher prevalence of AIDs in adults with SCD than in the general population.59,60 In fact, it is believed that AIDs are grossly under-diagnosed, under-reported, and under-estimated in SCD. This is because both conditions (AID and SCD) share many clinical and biological features such as inflammation, anaemia, pain, and multi-organ damage and dysfunction.56,61,62
Although autosplenectomy is a strong risk factor for AIDs, we believe that the risk associated with autosplenectomy in SCD patients is synergistically aggravated by additional risk factors for autoimmunity that are consistently associated with pathogenesis and/or management of SCD. For example, on the one hand autosplenectomy is associated with recurrent infections and chronic inflammation;63 and on the other hand, infections and inflammation play significant roles in the perturbation of self-tolerance within the concept of the so-called ‘second hit hypothesis’ in the cascade of events leading towards the development of AIDs.64 Hence, the high incidence of recurrent infections and chronic inflammation may be partly responsible for higher prevalence of AIDs in adult patients with SCD as compared with the general population.59,60 We thus reckon that the high prevalence of AIDs in SCD is multi-factorial, and is attributable to the combined, synergistic, and inter-related autoimmunity-inducing effects of tissue injury,65 recurrent infections and chronic inflammation,64 decreased self-tolerance due to autosplenectomy and defective splenic function,48-52 transfusion-associated chronic immune stimulation,56 release of hidden neo-antigenic epitopes during haemolytic episodes,66 haptenic effects of drugs and medications,67,68 and infection-induced molecular mimicry69,70 as appraised by different authors from different clinical perspectives. It is therefore not surprising that SCD is associated with the production of a wide range of autoantibodies that cause a myriad of AIDs in various solid organs and tissues, e.g., skeletal muscle (myasthenia gravis), smooth muscle (autoimmune hepatitis and cholangitis), nuclear proteins (SLE), joints (rheumatoid arthritis, RA), salivary and lacrimal glands (Sjogren’s syndrome), thyroid gland (autoimmune hypothyroidism), kidneys (autoimmune nephritis), skin (systemic sclerosis), haemostatic tissue components (anti-phospholipid syndrome), and several other components of the human tissues.56-60,71-73 In similarity with solid tissues, the blood is not spared by AIDs in SCD as several cases of primary and secondary autoimmune hemolytic anemia (AIHA) mediated by warm IgG autoantibodies and cold IgM autoantibodies were frequently reported among SCD patients.66-70,74-83
AIDs that affect solid tissues (typified by RA and SLE) are strongly associated with tissue damage (that releases more pro-inflammatory tDAMPs), immune activation, and inflammatory responses that lead to STERIN and ASTERIN, even in non-SCD patients.27 Comorbid AIDs would therefore aggravate SCD-associated STERIN and worsen steady state ASTERIN in patients with SCD. Moreover, comorbid AIDs that increase haemolysis (typified by AIHA) would further aggravate SCD-associated STERIN and ASTERIN by releasing more pro-inflammatory eDAMPs into circulation.13,45 It is thus imperative that AIDs in SCD are promptly identified and treated in order to ameliorate STERIN and ASTERIN and restore optimal steady state Hb concentration. Unfortunately, diagnosis of AIDs in SCD is often unduly delayed. This because comorbid AIDs in SCD patients are often ‘clinically disguised’ since they usually increase inflammation, worsen anaemia, raise blood viscosity, and aggravate vaso-occlusive morbidities, which often blend with the pre-existing symptoms of SCD.84 This scenario often leads to delayed diagnosis and increased risk of severe AID-associated organ damage in patients with SCD and comorbid AIDs. For examples, delayed diagnosis of RA would result in severe arthropathy in SCD patients with comorbid juvenile RA,85 and delayed diagnosis of SLE in SCD patients would lead to immune complex nephropathy.86-88 Hence, high index of suspicion is required for prompt diagnosis of AIDs in SCD.
The afore-discussed multi-faceted roles of tissue infarction, haemolysis, and AIDs in the pathogenesis of STERIN and ASTERIN vis-à-vis their adverse roles as erythropoiesis-suppressors and anaemia-aggravators in SCD call for a therapeutic strategy against STERIN and ASTERIN in SCD as described below.
1e. Therapeutic Strategy Against STERIN and ASTERIN in SCD
It can be deduced from the literature that sickling, tissue infarction, haemolysis and autoimmunity, and their pathophysiologic consequences can act individually and synergistically to generate STERIN and ASTERIN as highlighted in afore-presented paragraphs. Hence, therapeutic and prophylactic management of STERIN and ASTERIN in SCD can be achieved via separate or combined application of the following five therapeutic strategies, viz: 1. Reduce production of pro-inflammatory cytokines and molecules; 2. Block actions of pro-inflammatory cytokines and molecules; 3. Increase production of anti-inflammatory cytokines; 4. Administer natural modulators of inflammation; and/or 5. Apply immune suppressants to reduce production of autoantibodies as explained below.
1f. Management and Prevention of STERIN and ASTERIN in SCD: Hydroxyurea and Adjunct Drugs
The use of conventional non-steroidal inflammatory drugs (NSAIDs) for SCD is largely restricted to short-term control of acute STERIN-induced infarctive pain during vaso-occlusive crisis.2 Despite their anti-inflammatory effect, NSAIDs cannot be safely used for long-term control of chronic STERIN because of the risks of NSAID-related renal, gastrointestinal, and cardiovascular complications.2 However, chronic STERIN is a relentless aetiologic factor for ASTERIN in SCD, which should be tackled with long-term application of medications that are safer and more tolerable than conventional NSAIDs. The therapeutic objectives of tackling STERIN and ASTERIN in SCD is to minimize inflammation, improve EPO production, enhance red cell production, ameliorate anaemia, and attain an ‘optimal level’ of steady state Hb concentration. The roles of hydroxyurea and other drugs in achieving the aforementioned therapeutic objectives are explained below.
The success of hydroxyurea in treating SCD is due to its dual actions as an enhancer of HbF and a modulator of inflammation. First, hydroxyurea-induced elevation of HbF89 is associated with decrease in haemolysis and vaso-occlusive tissue damage,90 thereby reducing the production of pro-inflammatory eDAMPs and tDAMPs. In addition, hydroxyurea has been shown to decrease levels of pro-inflammatory cytokines with reciprocal increase in the levels of anti-inflammatory cytokines in SCD patients.91 Hence, it can be surmised that the overall improvement in steady state Hb concentration as seen in hydroxyurea-treated SCD patients is attributable to the combined effects of reduced levels of eDAMPs (due to reduced haemolysis),90 reduced levels of tDAMPs (due to alleviated vaso-occlusive tissue necrosis),90 decreased levels of pro-inflammatory cytokines,91 and increased levels of anti-inflammatory cytokines.91 The aforementioned multi-faceted effect of hydroxyurea against STERIN is evidenced by significant rise in levels of EPO in the plasma of SCD patients on hydroxyurea therapy;92,93 a scenario that would undoubtedly improve erythropoiesis, optimize compensation of SCD-related chronic haemolysis, down regulate ASTERIN, and ameliorate Hb concentrations among patients with SCD. While hydroxyurea is clinically proven to be a useful and safe drug against STERIN and other complications of SCD, the anti-inflammatory effect of hydroxyurea can be augmented by combined use of drugs that can inhibit sickling, haemolysis, vaso-occlusive tissue infarct, and/or oxidative stress. Such drugs include Mitapivat (pyruvate kinase inhibitor),94 Febuxostat (xanthine oxidase inhibitor),95 and L-glutamine (anti-oxidant),96 each of which can act synergistically with hydroxyurea to decrease the generation of pro-inflammatory mediators such as free radicals, cytokines, eDAMPs, and tDAMPs. Low levels of pro-inflammatory mediators would ultimately ameliorate steady state STERIN, enhance EPO production, mitigate ASTERIN, and improve anaemia in SCD. Potentially useful experimental interventions against STERIN in SCD include use of monoclonal antibodies (MoAbs) against pro-inflammatory cytokines,97 application of MoAbs against complement activation products in the lectin and alternate pathways,98 and administration of natural anti-inflammatory lipid mediators.99 Iron overloaded SCD patients should receive mandatory iron chelation therapy in order to reduce iron burden and its associated pro-inflammatory sequelae.44 Several iron chelators such as Deferiprone, Deferoxamine, and Deferasirox have already demonstrated significant anti-inflammatory effects in various clinical settings.100
SCD patients with comorbid AIDs present therapeutic challenge in the choice of immune suppressive drugs needed to decrease autoantibody production, down regulate STERIN and ASTERIN, and improve Hb concentrate. This is because steroidal immune suppressants (such as prednisolone) must be used cautiously in the treatment of AIDs in patients with SCD as they may trigger VOC.56 This may dictate the use of more expensive non-steroidal immune suppressants such as anti-TNF.56 As earlier mentioned, immunological studies strongly associate autosplenectomy48-52 with the high prevalence of AIDs in patients with SCD.59,60 It is thus predictable that the risk and incidence of AIDs in patients with SCD can be mitigated or prevented by the ‘spleen-preservation’ effect of hydroxyurea, if the drug administration is started in early childhood before the onset of autosplenectomy.101 Interestingly, hydroxyurea may even possibly ‘reverse’ splenic fibrosis and ‘restore’ splenic parenchymal and immune functions in older SCD patients with established autosplenectomy.102 These dual ‘spleen preservation’101 and ‘spleen restoration’102 effects of hydroxyurea would optimistically prevent the development of autosplenectomy-induced AIDs in patients with SCD on hydroxyurea.
2. SEPTIN and ASEPTIN in SCD: Aetiology and Pathogenesis
SCD patients’ susceptibility to infections is the main driver of SEPTIN, which subsequently aggravates pre-existing STERIN. Hence, pre-existing ASTERIN in SCD patients becomes aggravated by ASEPTIN during the course of comorbid infections as explained in subsequent paragraphs.
2a. Immune Dysfunctions: Susceptibility to Infections, SEPTIN, and ASEPTIN
SCD is associated with increased susceptibility to acute and chronic infections. This is partly due to functional hyposplenism and autosplenectomy resulting from recurrent vaso-occlusive infarcts within the spleen.15 Several other factors that predispose patients with SCD to infections include abnormalities of opsonization, antibody production, alternate complement pathway, and cell-mediated immunity.103-105 The range of SCD associated immune abnormalities basically determines the pattern of susceptibility to microbial agents in SCD as described below.
Hyposplenism and autosplenectomy predispose to severe infections with erythrocytotropic and erythrocytopathic infections (such as Malaria, Babesia, and Bartonella species) as well as encapsulated organisms (such as Haemophilus influenzae and Streptococcus pneumoniae).15,103 Low serum IgM levels, impaired opsonization, and abnormality of alternate complement pathway would further increase susceptibility to other common pathogens such as Mycoplasma pneumoniae, Salmonella typhimurium, Staphylococcus aureus, and Escherichia coli.103-106 Vaso-occlusive tissue necrosis is an important risk factor for infection. This is because necrotic tissue provides ‘sequestered’ foci of infections that are easily spread and propagated within the context of the pre-existing immunological dysfunction associated with SCD.107 Moreover, it had been proposed that the ability of salmonella to survive within hypo-perfused and poorly oxygenated necrotic tissues makes it a very common infective agent in patients with SCD.108 Indeed, it had been demonstrated that salmonella species can survive and flourish under the sanctuary of low oxygen tension.109 This is because hypoxia suppresses macrophage antimicrobial activity, and at the same time augment bacterial proliferation and virulence of salmonella species as demonstrated by a study on Salmonella typhimurium.109 Hyper-transfusion therapy for SCD associated disorders (such as stroke, priapism, acute chest syndrome, and chronic renal failure) causes transfusion-associated immune suppression (due to the effects of apoptotic and micro-particulate debris, and bioactive substances released from allogeneic leucocytes, platelets, and red cells),110 and increases the risks of transfusion transmissible infections (such as HIV and viral hepatitis)111 and iron overload.112 Transfusion-induced iron overload is a risk factor for infections with siderophilic bacteria such as Yersinia enterocolitica as reported in transfusion dependent patients, including those with SCD.113 Unfortunately, chelation therapy which is the gold standard for treating iron overload in SCD patients, is also an additional and independent risk factor for infection with Yersinia enterocolitica; the risk being higher with Deferoxamine than Deferiprone.114 Sickling-induced renal damage causes zincuria and zinc deficiency, which has been linked to impaired immunity and increased risk of infections in patients with SCD.115 Paradoxically, neutrophilia is a major haematological manifestation of SCD even in steady state116, nonetheless the neutrophils do not optimally protect the SCD patients from infections because of their phagocytic dysfunctions.117 Interestingly, the overall susceptibility of SCD patients to infection is partly determined by genetic polymorphism of the human leucocyte antigen (HLA) system.118 The HLA system has been shown to modulate susceptibility to bacteraemia in patients with SCD.118 While some alleles such as the HLA class II DRB1*15 have been shown to be protective, others like the HLA class II DQB1*03 occur significantly more in SCD patients with major infections, suggesting an increased susceptibility of the latter group to infections.118
2b. Infective Triggers of SEPTIN and ASEPTIN: Pathogen-Induced Acute Phase Reactions
Acute and chronic infections are major morbidities and triggers of SEPTIN in patients with SCD especially in low resource tropical regions,103 which carry the heaviest burden of SCD and wherein the majority of SCD patients reside.119 Infections in patients with SCD would generate pathogen associated molecular patterns (PAMPs).120,121 PAMPs subsequently trigger antimicrobial immune responses, generate pro-inflammatory hyper-cytokinemia, and ultimately establish SEPTIN.120,121 SEPTIN would pathophysiologically suppress production of EPO,26 and eventually cause ASEPTIN.27 It is therefore conceivable that SCD patients with frequent, acute, or chronic infections would have lower Hb concentrations in comparison with their uninfected counterparts as explained below.
Malaria is a frequent and prototype acute infection in hyposplenic and asplenic patients in general,122 including those with SCD in particular.123 Despite the fact that malarial anaemia is largely attributable to hyperhaemolysis of phagocytosed parasitized red cells,124 EPO-suppressive effect of pro-inflammatory cytokines-induced SEPTIN and ASEPTIN are additional components of malarial anaemia.125,126 Chronic infections are also important causes of SEPTIN and ASEPTIN even in non-SCD patient.27 Moreover, SCD patients are more vulnerable to EPO-suppressive effect of SEPTIN and erythropoiesis-suppressive effects of ASEPTIN than non-SCD individuals. This is because chronic SEPTIN in SCD would synergistically aggravate a pre-existing SCD-associated chronic STERIN,30,31 depress a pre-existing inappropriately low EPO levels,32,33 and cause greater inflammatory suppression of erythropoiesis resulting in severer anaemia via dual effects of ASTERIN and ASEPTIN.27 This scenario is typified by HIV and tuberculosis, both of which are prototype chronic infections that consistently cause SEPTIN and ASEPTIN even among persons without SCD.127,128 We reckon that patients with SCD have reduced expression of CCR5 and CCR7 chemokine receptors with a concomitant lower risk of acquisition and progression of HIV infection.129 Nonetheless, multi-transfused SCD patients in low resource African setting remain at high risk of HIV infection,130,131 as a result of poor transfusion safety.132 The separate and combined anaemia-aggravating effects of HIV and TB-induced SEPTIN and ASEPTIN in SCD patients had been classically revealed in three studies. The first study revealed that in comparison with their uninfected counterparts, HIV infected SCD patients tend to be more anaemic with lower Hb concentrations despite antiretroviral therapy.133 The second study revealed that in comparison with their uninfected counterparts, SCD patients with non HIV-associated pulmonary TB had lower levels of haematocrit coupled with sub-optimal reticulocytosis.134 And the third study had shown that dual co-infections with HIV and TB (each of which is an independent inducer of SEPTIN) in patients with SCD was associated with very high risk of ASEPTIN and transfusion dependence.135 The aforementioned three studies133-135 had confirmed that SEPTIN and ASEPTIN are negative modifiers and aggravators of anaemia in SCD, which by implication would undesirably decrease well being and increase the frequency of blood transfusion in infected patients with SCD. There is thus a need for therapeutic strategy against SEPTIN and ASEPTIN in SCD as described below.
2c. Therapeutic Strategy Against SEPTIN and ASEPTIN in SCD
Acute and chronic infections are the major triggers and sustainers of SEPTIN and ASEPTIN in SCD. Hence, therapeutic and prophylactic management of SEPTIN and ASEPTIN in SCD can be achieved via separate or combined application of the following three therapeutic strategies, viz: 1. Anti-microbial chemotherapy for active infections; 2. Anti-microbial chemoprophylaxis to prevent recurrence of infections; and 3. Immunoprophylaxis and/or use of hydroxyurea to boost patient resistance against pathogens as explained in subsequent paragraphs.
2d. Management and Prevention of SEPTIN and ASEPTIN in SCD: Chemotherapy, Chemoprophylaxis, Immunoprophylaxis, and Hydroxyurea.
It is obvious that SEPTIN is a frequent aetiologic factor for ASEPTIN in SCD.133-135 The therapeutic objective of tackling SEPTIN and ASEPTIN in SCD is to eradicate ongoing infections, improve EPO production, enhance red cell production, restore ‘pre-infection level’ of steady state Hb concentration in the short term, and forestall recurrence of infections in the long term as explained below.
In order to mitigate the EPO-suppressive effect of SEPTIN and boost Hb concentration of SCD patients, it is pertinent to combat acute and chronic infections. In the short-term, SCD patients presenting with any clinical feature of infection such fever should be promptly diagnosed and treated with appropriate antimicrobial chemotherapy using standardized protocols with the aims of eradicating the infection and abrogating any accompanying SEPTIN and ASEPTIN.136 For instance, it is particularly important to ensure that SCD patient with chronic infections due to HIV and/or TB are treated with anti-retroviral and anti-TB drugs with the aims of eradicating the infections,136 stopping the accompanying SEPTIN and ASEPTIN,127,128 and eventually restoring pre-infection steady state Hb concentration.133-135 Similarly, SCD patients with acute and frequent infections such as malaria123 should be promptly diagnosed and treated in order to achieve eradication of infection136 and mitigation of accompanying SEPTIN and ASEPTIN.125,126 Moreover, there is a long-term need for chemoprophylaxis and immunoprophylaxis against locally endemic infections among patients with SCD.136 For example, malaria is a major and recurrent morbidity in SCD patients who are resident in their native tropical malaria endemic regions.123 Hence, all SCD patients living in malaria endemic regions should be on lifelong anti-malarial chemoprophylaxis.137 Standard practice for preventing common bacterial diseases in SCD should include the initiation of chemoprophylaxis with daily penicillin at 2 months until the age of 5 years, by which time the patient would have completed polyvalent pneumococcal vaccines.103,104 In addition, a more comprehensive multi-vaccine immunoprophylaxis is highly recommended especially for SCD patients living in the tropics who should receive a full spectrum of routine vaccines consisting of BCG, Polio, Hepatitis-B, Diphtheria, Pertussis, Tetanus, Yellow fever, Measles and other vaccines against locally prevalent tropical pathogens such as Salmonella, Meningococci type-A and C, and Haemophilus influenzae type-B,138 as well as the RTS,S/AS01 malaria vaccine that was recently approved by the W.H.O.139 While SCD patients are encouraged to receive all locally relevant vaccines, caution should be exercised in HIV-infected SCD patients regarding administration of live vaccines vis-à-vis risk of reactivation and dissemination of attenuated vaccine-organisms.140
A recent and interesting study has revealed that hydroxyurea was associated with reduction in the risk and frequency of infections by multiple pathogens in children with SCD in a Ugandan series of patients.141 The study suggested that hydroxyurea might have reduced the frequency of infections by improving immune function via preservation and restoration of splenic functions.101,102 Moreover, the study suggested that hydroxyurea might also have anti-infective effect via direct antimicrobial activity as previous studies had demonstrated hydroxyurea-mediated in-vitro microbial killing of many pathogens, including Escherichia coli,142,143 and multiple parasites such as Leishmania species,144,145 Trypanosoma cruzi,144 Toxoplasma gondii,144,146 Babesia microti,147 and Plasmodium falciparum.148,149 It is therefore predictable that wider use of hydroxyurea in the tropics and malaria endemic countries would reduce the incidence of infections, SEPTIN, ASEPTIN, and other infection-associated anaemias in patients with SCD.
Conclusion
Hyper-inflammation in SCD has dual components; STERIN and SEPTIN. Accordingly, anaemia of inflammation in SCD has dual components; ASTERIN and ASEPTIN. On the one hand, ASTERIN is driven by STERIN, which is relentless and occurs throughout the course of SCD even in steady state. However, steady state STERIN and ASTERIN are often aggravated by infarctive and haemolytic crises, and/or comorbid autoimmune diseases. On the other hand, ASEPTIN is driven by SEPTIN, which occurs in SCD only during episodes of comorbid infections. Hence, during the course of infection, both ASTERIN and ASEPTIN act synergistically to worsen anaemia and increase the risk of red cell transfusion for patients with SCD. Anaemia in SCD is therefore not a mere reflection of quantitative loss of haemolysed sickled red cells. Rather, anaemia in SCD is significantly attributable to ASTERIN and ASEPTIN. Therefore, management and prevention of STERIN and SEPTIN are important strategies for up-regulating EPO production, de-suppressing erythropoietin function, enhancing red cell production, down-regulating ASTERIN and ASEPTIN, and ultimately augmenting Hb concentration and reducing transfusion requirement of patients with SCD.
References
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1993; 6: 215-262. DOI:10.1016/S0950-3536(05)80071-X.
- Darbari DS, Sheehan VA, Ballas SK. The vaso-occlusive pain crisis in sickle cell disease: Definition, pathophysiology, and management. Eur J Haematol 2020;105:237-246. DOI:10.1111/ejh.13430.
- Fleming AF, Storey J, Molineaux L, Iroko EA, Attai ED. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol 1979; 73: 161-172. DOI:10.1080/00034983.1979.11687243.
- Elguero E, Delicat-Loembet LM, Rougeron V, Arnathau C, Roche B, Becquart P, et al. Malaria continues to select for sickle cell trait in Central Africa. Proc Natl Acad Sci 2015;112:7051-4. DOI:10.1073/pnas.1505665112.
- Olatunji PO. Malaria and the sickle gene: Polymorphism balanced in favour of eradication. Ann Health Res 2018;4:88-96.
- Gong L, Parikh S, Rosenthal PJ, Greenhouse B. Biochemical and immunological mechanisms by which sickle cell trait protects against malaria. Malar J 2013;12:317. DOI:10.1186/1475-2875-12-317.
- Ogedegbe HO. ß-globin gene cluster haplotype analysis as a predictor of sickle cell disease severity. Lab Med 2007; 38:563-568. DOI:10.1309/XFMFQN614UR7GQGH..
- Ahmed SG, Ibrahim UA. Haemoglobin-S in sickle cell trait with papillary necrosis. Br J Haematol 2006;135:415-416. DOI:10.1111/j.1365-2141.2006.06318.x.
- Barbedo MM, McCurdy PR. Red cell life span in sickle cell trait. Acta Haematol 1974;51:339-343. DOI:10.1159/000208316.
- Fernando C, Mendis S, Upasena AP, Costa YJ, Williams HS, Moratuwagama D. Splenic syndrome in a young man at high altitude with undetected sickle cell trait. J Patient Exp 2018;5:153-155.
- Kasi PM, Patnaik MM, Peethambaram PP. Safety of pegfilgrastim (neulasta) in patients with sickle cell trait/anemia. Case Rep Hematol 2013;2013:146938. DOI:10.1155/2013/146938.
- Ahmed SG, Ibrahim UA. A compendium of pathophysiologic basis of etiologic risk factors for painful vaso-occlusive crisis in sickle cell disease. Niger J Basic Clin Sci 2017; 14: 57-77. DOI:10.4103/njbcs.njbcs_11_17.
- Mendonc R, Silveira AAA, Conran N. Red cell DAMPs and inflammation. Inflamm Res 2016; 65:665-678. DOI: 10.1007/s00011-016-0955-9.
- Domingos IF, Pereira-Martins DA, Sobreira MJVC, Oliveira RTD, Alagbe AE, Lanaro C, et al. High levels of proinflammatory cytokines IL-6 and IL-8 are associated with a poor clinical outcome in sickle cell anemia. Ann Hematol 2020;99: 947-953.
- Ahmed SG, Ibrahim UA. The role of acute and chronic splenic dysfunctions in aetiopathogenesis of anaemia in sickle cell disease: narrative review of hyperhaemolytic implications of autosplenectomy, autoimmunity, infections, and splenomegaly. Niger Health J 2023; 23:597-611. DOI:10.60787/tnhj.v23i2.680.
- Ahmed SG, Ibrahim UA. Pathophysiologic basis of haemolysis in patients with sickle cell disease in steady state and in hyperhaemolytic states: Aetiopathogenesis, management, and mitigation. Niger J Basic Clin Sci 2023;20:10-23. DOI:10.4103/njbcs.njbcs_55_22.
- Aboderin FI, Oduola T, Davison GM, Oguntibeju OO. A review of the relationship between the immune response, inflammation, oxidative stress, and the pathogenesis of sickle cell anaemia. Biomedicines 2023;11:2413. DOI:10.3390/biomedicines11092413.
- Pathare A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D. Cytokine profile of sickle cell disease in Oman. Am J Hematol 2004;77:323-328.
- Alagbe AE, Domingos IF, Adekile AD, Blotta MHSL, Santos MNN. Anti-inflammatory cytokines in sickle cell disease. Mol Biol Rep 2022;49:2433-2442.
- Sarray S, Saleh LR, Saldanha FL, Al-Habboubi HH, Mahdi N, Almawi WY. Serum IL-6, IL-10, and TNFa levels in pediatric sickle cell disease patients during vaso-occlusive crisis and steady state condition. Cytokine 2015;72:43-47. DOI:10.1016/j.cyto.2014.11.030.
- Hardy RA, Rached NA, Jones JA, Archer DR, Hyacinth HI. Role of age and neuroinflammation in the mechanism of cognitive deficits in sickle cell disease. Exp Biol Med 2021;246:106-120. DOI:10.1177/1535370220958011.
- Colella MP, Santana BA, Conran N, Tomazini V, Costa FF, Calado RT, et al. Telomere length correlates with disease severity and inflammation in sickle cell disease. Rev Bras Hematol Hemoter 2017;39:140-145.
- Masese RV, Ashley-Koch AE, Lê BM, Hatch D, Yang Q, Luyster F, et al. Associations between epigenetic age acceleration and psychoneurological symptoms in sickle cell disease. Blood 2023;142: 5263-5263.
- Lê BM, Hatch D, Yang Q, Shah N, Luyster FS, Garrett ME, et al. Characterizing epigenetic aging in an adult sickle cell disease cohort. Blood Adv 2024; 8:47-55.
- Helvaci MR, Sahan M, Sulhan A, Acik AF, Ocak A, Salaz S, et al. Accelerated atherosclerosis and digital clubbing in sickle cell diseases. Mid East J Fam Med 2016;14:10-16.
- Jelkmann WE, Fandrey J, Frede S, Pagel H. Inhibition of erythropoietin production by cytokines. Implications for the anemia involved in inflammatory states. Ann New York Acad Sci 1994;718:300-309.
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood 2019;133:40-50. DOI:10.1182/blood-2018-06-856500.
- Akinola NO, Stevens SME, Franklin IM, Nash GB, Stuart J. Subclinical ischaemic episodes during the steady state of sickle cell anaemia. J Clin Pathol. 1992; 45: 902-906. DOI:10.1136/jcp.45.10.902 .
- Sundd P, Ofori-Acquah SF. Sterile Inflammation in sickle cell disease. In: Mark T. Gladwin MT, Kato GJ, Novelli EM (eds): Sickle Cell Disease 2021; McGraw Hill, New York, NY. https://hemonc.mhmedical.com/content.aspx?bookid=3021§ionid=253922350.
- Singhal A, Thomas PW, Serjeant BE, Serjeant GR, Doherty JF, Raynes JG, et al. Is there an acute-phase response in steady-state sickle cell disease? Lancet 1993;341:651-653. DOI:10.1016/0140-6736(93)90418-G.
- Akohoue SA, Shankar S, Milne GL, Morrow J, Chen KY, Ajayi WU, et al. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr Res 2007;61:233-238.
- Pulte D, Nagalla S, Caro J. Erythropoietin levels in patients with sickle cell disease not in vaso-occlusive crisis. Blood 2012;120: 3242. DOI:10.1182/blood.V120.21.3242.3242.
- Sherwood JB, Goldwasser E, Chilcote R, Carmichael LD, Nagel RL. Sickle cell anemia patients have low erythropoietin levels for their degree of anemia. Blood 1986;67:46-49.
- Han Y, Gao C, Liu Y, Zhang H, Wang S, Zhao H, et al. Hemolysis-driven IFNa production impairs erythropoiesis by negatively regulating EPO signaling in sickle cell disease. Blood 2024;143:1018-1031. DOI:10.1182/blood.2023021658.
- Conran N, Belcher JD. Inflammation in sickle cell disease. Clin Hemorheol Microcirc 2018;68:263-299. DOI:10.3233/CH-189012.
- Hebbel RP, Belcher JD, Vercellotti GM. The multifaceted role of ischemia/reperfusion in sickle cell anemia. J Clin Invest 2020;130:1062-1072. DOI:10.1172/JCI133639.
- Tumburu L, Ghosh-Choudhary S, Seifuddin FT, Barbu EA, Yang S, Ahmad MM, at al. Circulating mitochondrial DNA is a pro-inflammatory DAMP in sickle cell disease. Blood 2021;137:3116-3126.
- Carvalho MOS, Santos TA, Reis JHO, Rocha LC, Cerqueira BAV, Luz NF, et al. Inflammatory mediators in sickle cell anaemia highlight the difference between steady state and crisis in paediatric patients. Bri J Haematol 2018;182:933-936. DOI:10.1111/bjh.14896.
- Gladwin MT, Ofori-Acquah SF. Erythroid DAMPs drive inflammation in SCD. Blood 2014;123:3689-3690. DOI:10.1182/blood-2014-03-563874.
- Biswal S, Rizwan H, Pal S, Sabnam S, Parida P, Arttatrana P. Oxidative stress, antioxidant capacity, biomolecule damage, and inflammation symptoms of sickle cell disease in children. Hematol 2019;24:1-9. DOI:10.1080/10245332.2018.1498441.
- Roumenina LT, Chadebech P, Bodivit G, Vieira-Martins P, Grunenwald A, Boudhabhay I, et al. Complement activation in sickle cell disease: Dependence on cell density, hemolysis, and modulation by hydroxyurea therapy. Am J Hematol 2020;95:456-464. DOI 10.1002/ajh.25742.
- Toledo SLD, Guedes JVM, Alpoim PN, Rios DRA, Pinheiro MD. Sickle cell disease: Hemostatic and inflammatory changes, and their interrelation. Clinica Chimica Acta 2019;493:129-137. DOI:10.1016/j.cca.2019.02.026.
- Inati A, Musallam KM, Wood JC, Taher AT. Iron overload indices rise linearly with transfusion rate in patients with sickle cell disease. Blood 2010;115:2980-2981.
- van Beers EJ, Yang Y, Raghavachari N, Tian X, Allen DT, Nichols JS, et al. Iron, inflammation, and early death in adults with sickle cell disease. Circ Res 2015;116:298-306. DOI:10.1161/CIRCRESAHA.116.304577.
- Nader E, Romana M, Connes P. The red blood cell-inflammation vicious circle in sickle cell disease. Front Immunol 2020;11:454.DOi:10.3389/fimmu.2020.00454.
- Loggetto SR. Sickle cell anemia: clinical diversity and beta S-globin haplotypes. Rev Bras Hematol Hemoter 2013;35:155-157. DOI:10.5581/1516-8484.20130048.
- Bandeira IC, Rocha LB, Barbosa MC, Elias DB, Querioz JA, Freitas MV, et al. Chronic inflammatory state in sickle cell anemia patients is associated with HBB(*)S haplotype. Cytokine 2014;65:217-221. DOI:10.1016/j.cyto.2013.10.009.
- McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MCI. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood 2011;117:5403-5412. DOI:10.1182/blood-2010-11-320028.
- Aliyu M, Zohora F, Saboor-Yaraghi AA. Spleen in innate and adaptive immunity regulation. AIMS Allergy and Immunology 2020;5:1-17. DOI:10.3934/Allergy.2021001.
- Kleiner-Baumgarten A, Schlaeffer F, Keynan A. Multiple autoimmune manifestations in splenectomized subject with HLA-B8. Arch Intern Med 1983;143:1987-1989. DOI:10.1001/archinte.1983.00350100171032.
- Balsalobre B, Hernández-Godoy J, Planelles D. Autoantibodies in splenectomized patients as a consequence of abdominal trauma. J Investig Allergol Clin Immunol 1992;2:91-95.
- Patel S, Kramer N, Rosenstein ED. Evolving connective tissue disease influenced by splenectomy: Beneath the sword of Dameshek. J Clin Rheumatol 2010;16:280-283. DOI:10.1097/RHU.0b013e3181eeb761.
- Babadoko AA, Ibinaye PO, Hassan A, Yusuf R, Ijei IP, Aiyekomogbon J, et al. Autosplenectomy of sickle cell disease in Zaria, Nigeria: an ultrasonographic assessment. Oman Med J 2012;27:121-123. DOI:10.5001/omj.2012.25.
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sickled spleen. Br J Haematol 2014;166:165-176. DOI:10.1111/bjh.12950.
- Wilson-Okoh DA, Nwauche CA, Ejele OA. Splenic changes in sickle cell anaemia. Niger J Med 2006;15:20-23.
- Li-Thiao-Te V, Uettwiller F, Quartier P, Lacaille F, Bader-Meunier B, Brousse V, et al. Coexistent sickle cell anemia and autoimmune disease in eight children: pitfalls and challenges. Pediatr Rheumatol 2018;16:5. DOI:10.1186/s12969-017-0221-x.
- Toly-Ndour C, Rouquette A-M, Obadia S, M’bappe P, Lionnet F, Hagege I, et al. High titers of autoantibodies in patients with sickle cell disease. J Rheumatol 2011; 38:302-309. DOI:10.3899/jrheum.100667.
- Quismorio Jr. P, Johnson C. Serum autoantibodies in patients with sickle cell anemia. Am J Med Sci 1984;287:13-15. DOI:10.1097/00000441-198401000-00003.
- Tang MW, Nur E, van Tuijn CFJ, Biemond BJ. Higher prevalence of autoimmune diseases in patients with sickle cell disease. Blood 2021;138:982.
- Igbineweka N, Darbari DS, Drasar ER, Steer S, Thein SL. Increased prevalence of autoimmunity and connective tissue diseases in sickle cell disease. J Gen Pract 2016;4:236. DOI:10.4172/2329-9126.1000236.
- Piccin A, O'Connor-Byrne N, Daves M, Lynch K, Farshbaf AD, Martin-Loeches I. Autoimmune disease and sickle cell anaemia: 'Intersecting pathways and differential diagnosis'. Bri J Haematol 2022;197:518-528. DOI:10.1111/bjh.18109.
- Vinit C, Guitton C, Benhaim P, Missud F, De Montalembert M, Amor L, et al. Auto-immune and inflammatory diseases in children with sickle cell disease: diagnostic and therapeutic issues. Ann Rheum Dis 2019;78:1999-1999.
- Sahu T, Pande B, Verma HK, Bhaskar LVKS, Sinha M, Sinha, R, et al. Infection and potential challenge of childhood mortality in sickle cell disease: A comprehensive review of the literature from a global perspective. Thalass Rep 2023; 13:206-229. DOI:10.3390/thalassrep13030019.
- Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol 2009;155:1-15.
- Steinman L. Interconnections between tissue injury, intermediary metabolism, autoimmunity, and chronic degeneration. Proc Am Thorac Soc 2006;3:484-488. DOI:10.1513/pats.200603-061MS.
- Motta I, Giannotta J, Ferraresi M, Barbullushi K, Revelli N, Graziadei G, et al. Autoimmune hemolytic anemia as a complication of congenital anemias. a case series and review of the literature. J Clin Med 2021;10:3439. DOI:10.3390/jcm10153439.
- Marques MB, Carr KD, Brumfield CG, Huang ST. Pregnant patient with sickle cell disease and Cefotetan-induced immune hemolysis. Lab Med 2000;31:541-543.
- Khurana M, Raj SS. Drug-induced hemolytic anemia: a fatal complication further under-recognized in sickle cell disease. Open J Blood Dis 2017;7:79-85. DOI:10.4236/ojbd.2017.73008.
- Fertu E, Haaser E, Dieudonné Y, Bauer S, Levy D, Rondeau-Lutz M, et al. Sickle cell disease in adulthood, mycoplasma, cold agglutinins: a report of 2 cases. La Revue de Médecine Interne 2016;37:A187. DOI:10.1016/j.revmed.2016.10.229.
- Inaba H, Geiger TL, Lasater OE, Wang WC. A case of hemoglobin SC disease with cold agglutinin-induced hemolysis. Am J Hematol 2005;78:37-40. DOI:10.1002/ajh.20244.
- Strauss J, Pardo V, Koss MN, Griswold W, McIntosh RM. Nephropathy associated with sickle cell anemia: An autologous immune complex nephritis: I. Studies on nature of glomerular-bound antibody and antigen identification in a patient with sickle cell disease and immune deposit glomerulonephritis. Am J Med 1975;58:382-387. DOI:10.1016/0002-9343(75)90604-X.
- Pardo V, Strauss J, Kramer H, Ozawa T, McIntosh RM. Nephropathy associated with sickle cell anemia: An autologous immune complex nephritis: II. Clinicopathologic study of seven patients. Am J Med 1975;59:650-659. DOI:10.1016/0002-9343(75)90226-0.
- Michel M, Habibi A, Godeau B, Bachir D, Lahary A, Galacteros F, et al. Characteristics and outcome of connective tissue diseases in patients with sickle-cell disease: report of 30 cases. Semin Arthritis Rheum 2008;38:228-240.
- Comenzo RL, Malachowski ME, Berkman EM. Clinical correlation of positive direct anti-globulin tests in patients with sickle cell disease. Immunohematology 1992;8:13-16.
- Chaplin H, Zarkowsky HS. Combined sickle cell disease and autoimmune hemolytic anemia. Arch Intern Med 1981;141:1091-1093. DOI:10.1001/archinte.1981.00340080127029.
- Maniatis A, Bertles JF, Wethers DL. Cold agglutinins and sickle-cell disease. Lancet 1977;1:50.
- Ward PCJ, Smith CM, White JG. An unusual morphologic finding in a case of sickle cell anemia with inter-current cold agglutinin syndrome. Am J Clin Pathol 1979;72:479-485.
- Bender T, Finn EM, Park C, Carden M. Unexplained hemolysis in a patient with sickle cell disease: DAT’s confusing. Abstract published at Hospital Medicine Meeting 2020. Abstract 1171. Journal of Hospital Medicine. https://shmabstracts.org/abstract/unexplained-hemolysis-in-a-patient-with-sickle-cell-disease-dats-confusing/.[Accessed: August 3, 2024].
- Rupani KV, Waksal J, Cytryn L, Naymagon L. Plasmodium falciparum-induced autoimmune hemolytic anemia in a pregnant patient with sickle cell disease. Am J Case Rep 2023;24:e938854. DOI:10.12659/AJCR.938854.
- Fuja C, Kothary V, Carll TC, Singh S, Mansfield P, Wool GD. Hyperhemolysis in a patient with sickle cell disease and recent SARS-CoV-2 infection, with complex auto- and alloantibody work-up, successfully treated with tocilizumab. Transfusion 2022;62:1446-1451. DOI:10.1111/trf.16932.
- Sakhalkar VS, Veillon DM, Cotelingam JD, McCaskill DM, Caldito GC, Hawthorne LM. Autoantibody formation in sickle cell disease patients receiving multiple blood transfusions. Blood 2005;106:3186. DOI:10.1182/blood.V106.11.3186.3186.
- Castellino SM, Combs MR, Zimmerman SA, Issitt PD, Ware RE. Erythrocyte autoantibodies in paediatric patients with sickle cell disease receiving transfusion therapy: frequency, characteristics and significance. Bri J Haematol 1999;104:189-194.
DOI:10.1046/j.1365-2141.1999.01127.x. - McNerney ME, Baron BW, Volchenboum SL, Papari M, KeithM, Williams K, et al. Development of warm auto- and allo-antibodies in a 3-year old boy with sickle cell haemoglobinopathy following his first transfusion of a single unit of red blood cells. Blood Transfus 2010;8:126-128. DOI:10.2450/2009.0105-09.
- Poutrel S, Boisson C, Nader E, Renoux C, Virot E, Catella J, et al. Clinical severity and blood rheology in patients with sickle cell anaemia and co-existing autoimmune disease Br J Haematol 2023;200:e28-e31. DOI:10.1111/bjh.18624.
- Nistala K, Murray KJ. Co-existent sickle cell disease and juvenile rheumatoid arthritis. Two cases with delayed diagnosis and severe destructive arthropathy. J Rheumatol 2001;28:2125-2128.
- Idris AB, Abdulgayoom A, Mudawi E, El Hassan AM, Elamin EM, Lamyaa-El Hassan LAM. Coexistence of sickle cell nephropathy and lupus nephritis in a Sudanese child. Saudi J Kidney Dis Transpl 2015;26:584-588.
- Kanodia KV, Vanikar AV, Goplani KR, Gupta SB, Trivedi HL. Sickle cell nephropathy with diffuse proliferative lupus nephritis: a case report. Diagnostic Pathol 2008;3:9. DOI:10.1186/1746-1596-3-9.
- Minocha V, Rana F. Lupus nephritis in a patient with sickle cell disease. Case Rep Hematol 2013; Article ID: 907950. DOI:10.1155/2013/907950.
- Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest 1984;74:652-656. DOI:10.1172/JCI111464.
- Keikhaei B, Yousefi H, Bahadoram M. Hydroxyurea: clinical and hematological effects in patients with sickle cell anemia. Glob J Health Sci 2015;8:252-256. DOI:10.5539/gjhs.v8n3p252.
- Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol 2009;85:235-242. DOI:10.1189/jlb.0708445.
- Papassotiriou I, Voskaridou E, Stamoulakatou A, Loukopoulos D. Increased erythropoietin level induced by hydroxyurea treatment of sickle cell patients. Hematol J 2000;1:295-300. DOI:10.1038/sj.thj.6200049.
- Zhang X, Zhang W, Shah B, Miasnikova G, Sergueeva A, Ammosova T, et al. Hydroxyurea treatment is associated with elevated serum erythropoietin concentration but suppressed global hypoxic transcriptional responses in sickle cell disease. Blood 2015;126:3380. DOI:10.1182/blood.V126.23.3380.3380.
- Rab MAE, Bos J, van Oirschot BA. Decreased activity and stability of pyruvate kinase in sickle cell disease: A novel target for Mitapivat therapy. Blood 2021;137:2997-3001.
- Schmidt HM, Wood KC, Lewis SE, Hahn SA, Williams XM, McMahon B, et al. Xanthine oxidase drives hemolysis and vascular malfunction in sickle cell disease. Arterioscler Thromb Vasc Biol 2021;41:769-782.
- Pavitra E, Acharya RK, Gupta VK, Verma HK, Kang H, Lee J-H, et al. Impacts of oxidative stress and anti-oxidants on the development, pathogenesis, and therapy of sickle cell disease: A comprehensive review. Biomed Pharmacother 2024;176:116849. DOI:10.1016/j.biopha.2024.116849.
- Rees DC, Kilinc Y, Unal S, Dampier C, Pace BS, Banu Kaya B, et al. A randomized, placebo-controlled, double-blind trial of canakinumab in children and young adults with sickle cell anemia. Blood 2022;139:2642-2652.
- Belcher JD, Nguyen J, Chen C, Abdulla F, Conglin R, Ivy ZK, et al. MASP-2 and MASP-3 inhibitors block complement activation, inflammation, and microvascular stasis in a murine model of vaso-occlusion in sickle cell disease. Translational Res 2022;249:1-12. DOI:10.1016/j.trsl.2022.06.018.
- Matte A, Recchiuti A, Federti E, Koehl B, Mintz T, El Nemer W, et al. Resolution of sickle cell disease associated inflammation and tissue damage with 17R-Resolvin D1. Blood 2019;133:252-265. DOI:10.1182/blood-2018-07-865378.
- Di Paola A, Tortora C, Argenziano M, Marrapodi MM, Rossi F Emerging roles of the iron chelators in inflammation. Int J Mol Sci 2022;23:7977. DOI:10.3390/ijms23147977.
- Nottage KA, Ware RE, Winter B, Smeltzer M, Wang WC, Hankins JS, et al. Predictors of splenic function preservation in children with sickle cell anaemia treated with hydroxyurea. Eur J Haematol 2014;93:377-383. DOI:10.1111/ejh.12361.
- Claster S, Vichinsky E. First report of reversal of organ dysfunction in sickle cell anemia by the use of hydroxyurea: Splenic regeneration. Blood 1996; 88:1951-1953.
- Cannas G, Merazga S, Virot E. Sickle cell disease and infections in high- and low-income countries. Mediterr J Hematol Infect Dis 2019; 11: 02019042. DOI:10.4084/mjhid.2019.042.
- Ochocinski D, Dalal M, Black LV, Carr S, Lew J, Sullivan K, et al. Life-threatening infectious complications in sickle cell disease: A concise narrative review. Front Pediatr 2020;8:38. DOI:10.3389/fped.
- Salawu L, Orimolade EA, Durosinmi MA. Immunohaematological characteristics of Nigerian sickle cell disease patients in asymptomatic steady state. Eur J Gen Med 2009;6:170-174.
- Dieye TN, Ndiaye O, Ndiaye BO. Complement and serum immunoglobulins in homozygous and heterozygous sickle cell anaemia in Senegal. Dakar Med 1999;44:175-179.
- Blacksin MF, Finzel KC, Benevenia J. Osteomyelitis originating in and around bone infarcts giant sequestrum phenomena. Am J Roentgenol 2001;176:387-391.
- Elbashier AM, Al-Salem AH, Aljama A. Salmonella as a causative organism of various infections in patients with sickle cell disease. Ann Saudi Med 2003;23:358-362. DOI:10.5144/0256-4947.2003.358.
- Jennewein J, Matuszak J, Walter S, Felmy B, Gendera K, Schatz V, et al. Low-oxygen tensions found in Salmonella-infected gut tissue boost Salmonella replication in macrophages by impairing antimicrobial activity and augmenting Salmonella virulence. Cell Microbiol 2015; 17:1833-1847. DOI:10.1111/cmi.12476.
- Youssef LA, Spitalnik SL. Transfusion-related immunomodulation: a reappraisal. Curr Opin Hematol 2017;24:551-557. DOI:10.1097/MOH.0000000000000376.
- Bakarey AS, Akinboade IO, Aken'Ova YA. Transmission transmissible hepatitis B virus markers of infection among sickle cell disease patients receiving care at a tertiary health facility in Ibadan, southwest Nigeria. J Immunoassay Immunochem 2018;39:416-427. DOI:10.1080/15321819.2018.1495649.
- Sekongo YM, Saydou K, Boidy K, Sidonie K, Kadidja K, Parfait N, et al.. Assessment of the risk of hemochromatosis in polytransfused sickle cell patients at the Abidjan transfusion therapy unit. Am J Biomed Life Sci 2020; 8:20-24. DOI:10.11648/j.ajbls.20200801.15.
- Blei F, Puder DP. Yersinia enterocolitica bacteremia in a chronically transfused patient with sickle cell anemia: case report and review of the literature. J Pediatr Hematol Oncol 1993; 15:430-434.
- Lesic B, Foulon J, Carniel E. Comparison of the effects of Deferiprone versus Deferoxamine on growth and virulence of Yersinia enterocolitica. Antimicrob Chemother 2002; 46:1741-1745. DOI:10.1128/AAC.46.6.1741-1745.2002.
- Bao B, Prasad AS, Beck FW, Snell D, Suneja A, Sarkar FH, et al. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl Res 2008;152:67-80.
- Iheanacho OE. Haematological parameters of adult and paediatric subjects with sickle cell disease in steady state, in Benin City, Nigeria. Int Blood Res Rev 2015;3:171-177.
- Humbert JR, Winsor EL, Githens JM, Schmitz JB. Neutrophil dysfunctions in sickle cell disease. Biomed Pharmacother 1990;44:153-158. DOI:10.1016/0753-3322(90)90002-Q.
- Tamouza R, Neonato MG, Busson M, Marzais F, Girot R, Labie D, et al. Infectious complications in sickle cell disease are influenced by HLA class II alleles. Hum Immunol 2002;63:194-199.
- Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med 2013;10: e1001484.
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009;22:240-273. DOI:10.1128/CMR.00046-08.
- Puerta-Guardo H. From pathogenic infections to inflammation and disease - the tumultuous road of the ‘cytokine storm’. Front Cell Infect Microbiol 2022; 11:827151. DOI:10.3389/fcimb.2021.827151.
- Ghosh D, Stumhofer JS. The spleen: “epicenter” in malaria infection and immunity. J Leukoc Biol 2021;110:753-769. DOI:10.1002/JLB.4RI1020-713R.
- Mkombachepa M, Khamis B, Rwegasira G, Urio F, Makani J, Luzzatto L. High incidence of malaria in patients with sickle cell disease. Am J Hematol 2022;97:E380-E381. DOI:10.1002/ajh.26676.
- Montgomery CP, Hoehn KS, Glikman D. Hyperhemolytic crisis caused by severe P. falciparum malaria in a boy with sickle cell anemia. Crit Care Med 2006; 34:A164. DOI:10.1097/00003246-200612002-00569.
- McDevitt MA, Xie J, Gordeuk V, Bucala R. The anemia of malaria infection: role of inflammatory cytokines. Curr Hematol Rep 2004;3:97-106.
- El Hassan AMA, Saeed AM, Fandrey J, Jelkmann W. Decreased erythropoietin response in Plasmodium falciparum malaria-associated anemia. Eur J Haematol 1997;59:299-304. DOI:10.1111/j.1600-0609.1997.tb01690.x.
- Enumah CL, Nwauche C, Okerengwo A. Anaemia and pro-inflammatory cytokines in human immunodeficiency virus infection. Int J Immunol 2016;4:13-18. DOI:10.11648/J.IJI.20160403.12.
- Gil-Santana L, Cruz LAB, Arriaga MB, Miranda PFC, Fukutani KF, Silveira-Mattos PS, et al. Tuberculosis-associated anemia is linked to a distinct inflammatory profile that persists after initiation of anti-tubercular therapy. Sci Rep 2019;9:1381. DOI:10.1038/s41598-018-37860-5.
- Kelly S, Jacobs ES, Stone M, Keating SM, Lee T-H, Chafets D, et al. Influence of sickle cell disease on susceptibility to HIV infection. PLoS ONE 2020;15:e0218880. DOI:10.1371/journal.pone.0218880.
- Françoise NS, Carole E, Evelyne N, Haman Z, Dora M. Prevalence of HIV seropositivity among sickle cell disease patients at the Yaoundé Central Hospital. Health Sci. Dis 2013;14:1-5.
- Ubesie AC, Emodi IJ, Ikefuna AN, Ilechukwu GC, Ilechukwu G. Prevalence of human immunodeficiency virus transmission among transfused children with sickle cell anemia in Enugu Nigeria. Ann Med Health Sci Res 2012;2:109-113. DOI:10.4103/2141-9248.105655.
- Ahmed SG. Transfusion services in tropical Africa: Challenges and prospects from the Nigerian perspective. Niger J Haematol 2022;3:1-17.
- Belisário AR, Blatyta PF, Vivanco D, Oliveira CDL, Carneiro-Proietti AB, Sabino EC, et al. Association of HIV infection with clinical and laboratory characteristics of sickle cell disease. BMC Infect Dis 2020;20:638. DOI:10.1186/s12879-020-05366-z.
- Ahmed SG, Bukar AA, Jolayemi B. Hematological indices of sickle cell anaemia patients with pulmonary tuberculosis in northern Nigeria. Mediterr J Hematol Infect Dis 2010;2:e2010014. DOI:10.4084/MJHID.2010.014.
- Sani A, Kumo BA, Kasim PM, Haruna MM. Transfusion dependent anemia in a patient with sickle cell disease as well as HIV-associated tuberculosis. Sub-Saharan Afr J Med 2017;4:52-55. DOI:10.4103/ssajm.ssajm_8_17.
- Sobota A, Sabharwal V, Fonebi G, Steinberg M. How we prevent and manage infection in sickle cell disease. Br J Haematol 2015;170:757-767. DOI:10.1111/bjh.13526.
- Oniyangi O, Omari AA. Malaria chemoprophylaxis in sickle cell disease. Cochrane Database Syst Rev 2019;11. DOI:10.1002/14651858.CD3489.pub2.
- Nease EK, Nield LS. Immunizations in the Child with Sickle Cell Disease. In: Kamat D., Frei-Jones M. (eds) Benign Hematologic Disorders in Children, 2021;pp 405-415. Springer, Cham. DOI:10.1007/978-3-030-49980-8_28.
- Drysdale C, Kelleher K. WHO recommends ground breaking malaria vaccine for children at risk. Geneva: WHO; 2021.https://www.who.int/news/item/06-10-2021-whorecommends-groundbreaking-malaria-vaccine-forchildren-at-risk. (Accessed: August 3, 2024).
- Le Corre N, Autran B. Vaccination in HIV-infected individuals. Future Virol 2012;7:85-102. DOI:10.2217/fvl.11.128.
- Namazzi R, Bond C, Conroy AL, Datta D, Tagoola A, Goings MJ, et al. Hydroxyurea reduces infections in children with sickle cell anemia in Uganda. Blood 2024;143:1425-1428. DOI:10.1182/blood.2023021575.
- Rosenkranz HS. Some biological effects of carbamoyloxyurea, an oxidation product of hydroxyurea. J Bacteriol 1970;102:20-23.
- Kuong KJ, Kuzminov A. Cyanide, peroxide and nitric oxide formation in solutions of hydroxyurea causes cellular toxicity and may contribute to its therapeutic potency. J Mol Biol 2009;390:845-862.
- Melo EJ, Beiral HJ. Effect of hydroxyurea on the intracellular multiplication of Toxoplasma gondii, Leishmania amazonensis and Trypanosoma cruzi. Braz J Med Biol Res 2003;36:65-69.
- Martinez-Rojano H, Mancilla-Ramirez J, Qui˜nonez-Diaz L, Galindo-Sevilla N. Activity of hydroxyurea against Leishmania mexicana. Antimicrob Agents Chemother 2008;52:3642-3647.
- Carvalho CS, Melo EJ. Acidification of the parasitophorous vacuole containing Toxoplasma gondii in the presence of hydroxyurea. An Acad Bras Cienc 2006;78:475-484.
- El-Saber Batiha G, Magdy Beshbishy A, Stephen Adeyemi O, Nadwa E, Rashwan E, Yokoyama N, et al. Safety and efficacy of hydroxyurea and eflornithine against most blood parasites: Babesia and Theileria. PLoS One 2020;15:e0228996.
- Pino P, Taoufiq Z, Brun M, Tefit M, Franetic JF, Ciceron L, et al. Effects of hydroxyurea on malaria, parasite growth and adhesion in experimental models. Parasite Immunol 2006;28:675-680.
- Safeukui I, Ware RE, Mohandas N, Haldar K. Simultaneous adjunctive treatment of malaria and its coevolved genetic disorder sickle cell anemia. Blood Adv 2023;7:5970-5981.