Recurrent subdural hemorrhages and evaluation of annual prostate screening
Iheanacho J1, Uwanuruochi K2
Abstract
We report a 76-year old man who had cerebellar hemorrhage and recurrent subdural hematoma. Investigations revealed that these were metastases from cancer of the prostate. We discuss the need for annual prostate cancer assessment in our environment.
Key words: Cancer of the prostate, Prostatic specific antigen, subdural hematoma
Key Message: The indications for annual examination of the prostatic specific antigen in elderly men should be appreciated.
Case Report
MU is a 76-year-old man from Lagos state came to the clinic with generalized weakness, poor appetite and weakness of right limbs. Recent medical history had been eventful. Six months before, he had subdural hematoma (Figure 1) for which he underwent cranial burr hole for drainage. Two months later, he developed right-sided weakness of the face and the right limbs, being managed for cerebellar hemorrhagic stroke with good recovery after three days. He was also hypertensive and diabetic, and had been treated for benign prostatic hyperplasia.
On presentation he was afebrile, not pale, not dehydrated, with memory deficits and reduced movement of right limbs, drowsy. His pupils were 2mm diameter, equal and reactive. Muscle tone was normal and power was reduced in right limbs. Reflexes were also normal and plantars flexor. He showed past-pointing and left sided dysdiadochinesia. There was no opthalmoplegia, the neck was supple. His blood pressure was 150/80 mmHg, and pulse 72/minute. His respiratory rate was 20/minute and oxygen saturation was 94%. Investigations showed normal clotting profile and bleeding time, haemoglobin 10.4 g/dl, platelet count 253 ×109/L, serum creatinine 77µmol/L and estimated glomerular filtration rate was 90ml/min/1.73m2. HDL-Cholesterol was 53 mg/dl, LDL-Cholesterol 37mg/dl, total cholesterol 97mg/dl, triglyceride 83mg/dl.
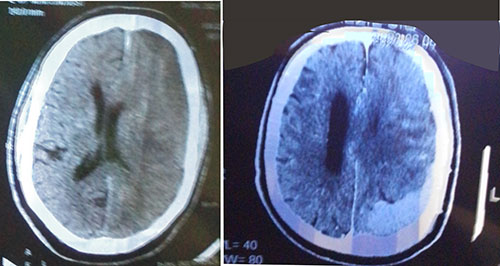
Figure 1: Acute left subdural hematoma (left) and massive left subdural hematoma over the parieto-occipital region, with midline shift (right)
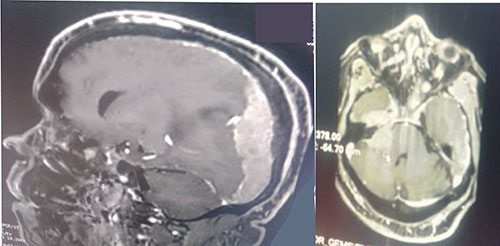
Figure 2: Extensive left dural-based mass, effacement of sulci and compression of ventricles
Non-constrast computer-tomography scan of the chest showed largely clear lung with scanty basal patchy densities on the right, some enlarged mediastinal lymph nodes, sclerotic changes in thoracic vertebrae, hydronephrotic left kidney. The features raised suspicion of cancer of the prostate with left ureteric obstruction and sclerotic spinal metastases. A computer tomography brain scan showed a massive left subdural hematoma over the parieto-occipital region, with midline shift and a hyperdense lesion on the right fronto-temporal lobe, and hydrocephalus (Figure 1). Cranio-cerebral magnetic resonance imaging showed an extensive left dural-based mass, mass effect with effacement of ipsilateral sulci and gyri, with compression of lateral and third ventricles, compression of thalamus and brainstem with 10.2mm shift of midline to contralateral side, copious perilesional edema and homogenous enhancement on contrast administration (Figure 2). An impression of multifocal extra-axial brain metastases from an occult primary and leptomeningeal metastases from prostate cancer being suspected. Digital rectal examination showed a hard prostate and Prostatic specific antigen (PSA) was more than 100 ng/ml. On admission he was given intravenous normal saline 1litre, 12hourly, intravenous 20% mannitol, 150mls over 20minutes, eight-hourly for 72 hours, intravenous dexamethasone 8mg 8-hourly for 72 hours and tablet losartan 25mg daily. Following diagnosis of cancer of the prostate, he was placed on chemotherapy by the urologists, experiencing marked clinical improvement in alertness and cognition, and power in the limbs.
Discussion
Prostate cancer screening is controversial.1 Our patient had two episodes of subdural haemorrhage before the underlying cancer of prostate was suspected and unravelled. PSA and digital rectal examination may detect aggressive cancers early, leading to improved outcomes. An annual PSA screening may have detected prostate cancer and prevented the metastases he developed. However, regular PSA screening has been associated with false-positivity and unnecessary treatment. It is important to ensure that guidelines being utilized are safe and effective.2 The finding of PSA >4 ng/ml in 11.15% of a Nigerian population reveals the need for greater awareness and measures to increase early detection.3 However, the value and validity of established PSA reference ranges and cutoff of 'normal' still need to be established. Investigators found that men who underwent annual prostate cancer screening had a higher incidence of prostate cancer than unscreened men but about the same rate of deaths from the disease.4 This suggests that many men were treated for prostate cancers that would not have been detected in their lifetime without screening. Consequently, some men were exposed unnecessarily to the potential harms of treatment.
Workers in the United Kingdom found no difference in prostate cancer mortality between those that had and did not have prostate cancer screening.5 A systematic review and meta-analysis of randomized controlled trials concluded that prostatic specific antigen screening for prostate cancer leads to a small reduction in prostate cancer mortality over 10 years but does not affect overall mortality.6 Following a PSA test many patients would have a false-positive biopsy, of those diagnosed, many would be treated with surgery or radiation, some developing complications such as urinary incontinence and sexual dysfunction, whereas the mortality benefit appears slight-1 death avoided for 570 men screened.7 A contradicting claim of about 50% reduction in prostate cancer-specific mortality from PSA-screened groups compared to identical but unscreened populations has however been made.8 This calls for further studies.
Some workers have suggested a screening interval of two years for low risk patients, viz non-Africans, nil family history of prostate cancer, PSA <1 ng/mL at 40 years, and <2 ng/mL at 60 years.9 An annual prostate cancer screening has been recommended for informed, selected individuals between age 45 to 75, especially with high-risk factors such as African descent, positive family history, family history of multiple cancers or Lynch syndrome, and high-risk genomic mutation such as BRCA1 or BRCA2 mutations.10
Though mannitol use has been linked to expansion of extradural hematoma, emergency preoperative high dose mannitol administration was associated with improved clinical outcomes for patients with acute subdural hematomas.11
References
- Baratedi WM, Tshiamo WB, Mogobe KD, McFarland DM. Barriers to Prostate Cancer Screening by Men in Sub-Saharan Africa: An Integrated Review. J Nurs Scholarsh. 2020 Jan;52(1):85-94. doi: 10.1111/jnu.12529. Epub 2019 Nov 15. PMID: 31733043.
- Makau-Barasa LK, Manirakiza A, Carvalho AL, Rebbeck TR. Prostate Cancer Screening, Diagnostic, Treatment Procedures and Costs in Sub-Saharan Africa: A Situational Analysis. Cancer Control. 2022 Jan-Dec;29:10732748221084932. doi: 10.1177/10732748221084932. PMID: 35350915; PMCID: PMC8973068.
- Akinremi T, Adeniyi A, Olutunde A, Oduniyi A, Ogo C. Need for and relevance of prostate cancer screening in Nigeria. Ecancermedicalscience. 2014 Aug 28;8:457. doi: 10.3332/ecancer.2014.457. PMID: 25228913; PMCID: PMC4154944.
- Pinsky PF, Prorok PC, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer 2017; 123(4):592–599.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity Prostatic surface antigen -based screening intervention on prostate cancer mortality: The CAP randomized clinical trial. JAMA 2018; 319(9):883–895.
- Ilic D, Djulbegovic M, Jung JH, Hwang EC, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. British Medical Journal 2018; 362:k3519.
- Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr follow-up of the European Randomized Study of Screening for Prostate Cancer. European Urology 2019; 76(1):43–51.
- Van Poppel H, Albreht T, Basu P, Hogenhout R, Collen S, Roobol M. Serum PSA-based early detection of prostate cancer in Europe and globally: past, present and future. Nat Rev Urol. 2022 Sep;19(9):562-572.
- Gelfond J, Choate K, Ankerst DP, Hernandez J, Leach RJ, Thompson IM. Intermediate-Term Risk of Prostate Cancer is Directly Related to Baseline Prostate Specific Antigen: Implications for Reducing the Burden of Prostate Specific Antigen Screening. J Urol. 2015 Jul;194(1):46-51.
- Tikkinen KAO, Dahm P, Lytvyn L, Heen AF, Vernooij RWM, Siemieniuk RAC, Wheeler R, Vaughan B, Fobuzi AC, Blanker MH, Junod N, Sommer J, Stirnemann J, Yoshimura M, Auer R, MacDonald H, Guyatt G, Vandvik PO, Agoritsas T. Prostate cancer screening with prostate-specific antigen (PSA) test: a clinical practice guideline. BMJ. 2018 Sep 5;362:k3581. doi: 10.1136/bmj.k3581. PMID: 30185545; PMCID: PMC6283372.
- Cruz J, Minoja G, Okuchi K. Improving clinical outcomes from acute subdural hematomas with the emergency preoperative administration of high doses of mannitol: a randomized trial. Neurosurgery. 2001 Oct;49(4):864-71. doi: 10.1097/00006123-200110000-00016. PMID: 11564247.