Physicians’ awareness and use of drug-drug interactions software: A preliminary study
Oriaifo OG1, Opadeyi AO2,3, Isah AO2,3
Abstract
Background: Drug-drug interactions software is used as a tool to investigate clinically harmful interactions. Prescribers need to be aware of these tools to improve the quality of drug prescriptions, thus ensuring better patient care. Drug-drug interactions are a common avoidable type of adverse drug reactions, which can have detrimental effects on patients in the form of drug toxicity
Objective: To determine the awareness of prescribers and the use of drug-drug interaction software
Method: The awareness of physicians of drug interaction software was assessed. A self-administered questionnaire was administered to all resident physicians in the Department of Internal Medicine, University of Benin Teaching Hospital to assess their awareness and use of the drug-drug interaction software. The information sought includes sociodemographic characteristics, Physician’s awareness, and use of drug interaction software. The results are presented descriptively.
Results: Thirty-four medical residents filled out the self-administered questionnaire. The number of physicians aware of drug-drug interactions software was 30 (82.2%) for Medscape, Drugs.com 21 (61.8%), Lexi-interact 4(11.8%), and 3 (8.8%) for Epocrates. Medscape interaction checker was most used by 26 (76.5%) while Micromedex was least used by one (2.9%) medical resident.
Conclusion: Physicians were largely aware of and used Medscape.
Keywords: Physician, Software, drug interaction, drug-drug interaction, awareness
Introduction
Drug-drug Interactions (DDI) software is mainly used in the prevention of adverse drug events that arise when multiple medicines are used. They provide timely information relating to the risk and possible harms that may occur when the properties of a drug alter another.1 They also provide information that may prevent the prescription or use of these medicines in the same setting.
Drugs are known to interact with others in various ways; pharmaceutical, pharmacokinetic interaction, and pharmacodynamics interactions.2
Four hundred and two potential adverse drug events including deaths and disabilities3 were prevented after prescribers were issued DDI alerts. Drug-drug interactions can however be such as the combination of amiloride and hydrochlorothiazide.
Drug information is often sought by health care professionals, with increased emphasis on electronic sources as shown in a study where both prescribers and pharmacists most commonly used electronic references, such as Micromedex®, the Internet, personal digital assistants (PDAs), and UpToDate®. Pharmacists became the next most often used source of DDI information for prescribers, while printed materials including Facts & Comparisons and the American Hospital Formulary Service were used by pharmacists.4 Assessing the need for and possible effectiveness of automated DDI warnings system in lowering DDIs may involve testing prescribers' capacity to identify potential DDIs without the use of drug references5 The source of information, in addition to knowledge of Potential drug-drug interactions, adds to the rational use of medications. For instance, improper medication use may occur if doctors depend on drug information provided by pharmaceutical corporations rather than evidence-based recommendations.6 An earlier study in the same setting had shown that junior doctors do use the internet to check drug-drug interaction using Google, Medscape, and Wikipedia mostly.7
The development of preventable adverse drug reactions in a hospital setting necessitates providing a systematic approach to reducing these reactions. Awareness of potential drug-drug interactions that may occur will be useful in our resource-constrained environment to reduce the healthcare cost that accounts for the development of these ADRs. The negative impact of DDI can be reduced or avoided when doctors are familiar with the use of drug interaction checkers or have computerized warning systems.
It is therefore imperative to mitigate the development of preventable ADE from drug–drug interactions. The use of predictive tools (both electronic and print formats) has been demonstrated to reduce these drug-drug interactions.8 The availability and accessibility of such DDI software may ease the physicians’ busy schedules. It is however uncertain if these tools are frequently accessed in Nigeria. This study will determine the physician’s awareness and use of drug-drug interaction software
Method
This cross-sectional study was carried out at the University of Benin Teaching Hospital, Benin City (UBTH), Edo State. UBTH is a tertiary hospital with 860 beds, it was founded with a tripartite mandate of training, clinical services, and research. It also functions as a referral center for primary and secondary health institutions in Edo and its neighboring states (Ondo, Delta, Bayelsa, and Kogi).
The study population was Physicians (Registrar and Senior Registrars) in Internal Medicine at, the University of Benin Teaching Hospital, Benin City, Nigeria.
All resident doctors in internal medicine (Registrars and Senior Registrar) were approached to participate in the study on awareness of drug-drug interactions software.
Total population sampling was used as the sampling technique for the study on Physicians’ (Senior Registrars and Registrars) awareness of drug-drug interactions tools except for Physicians who were on their annual leave.
A self-administered questionnaire was administered to all resident physicians in the Department of Internal Medicine, University of Benin Teaching Hospital to assess their awareness and use of the drug-drug interaction software. The information sought included sociodemographic characteristics, Physician’s awareness, and use of drug interaction software.
For all statistical analysis, the Statistical Package for the Social Sciences version 24 (IBM) was used. Frequencies/percentages were determined for the categorical variables, and continuous variables were represented as mean ±SD.
Results
Socio-demographic characteristics
A total of 34 resident physicians in the internal medicine department participated in the study. The median age was 35 (range 27-50) and the majority 25 (73.5%) were aged between the ages of 31-40, Eighteen (52.9%) were male. This is shown in detail in Table 1.
Table 1: Socio-demographic characteristics of resident doctors in the Internal Medicine Department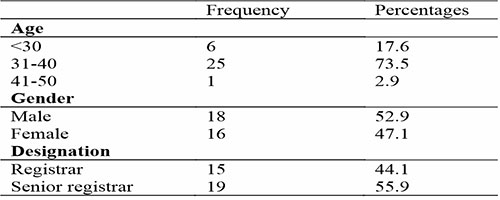
Awareness and use of drug-drug interactions checker
Regarding the awareness of medical residents on drug-drug interactions software, 30 (82.2%) were aware of Medscape. 21 (61.8%) were also aware of drugs.com. Few doctors 3 (8.8%) were aware of Epocrates. Medscape was most used by 26 (76.5%) while Micromedex was least used by one (2.9) medical resident (Table 2).
Table 2: Awareness and use of drug-drug interactions checker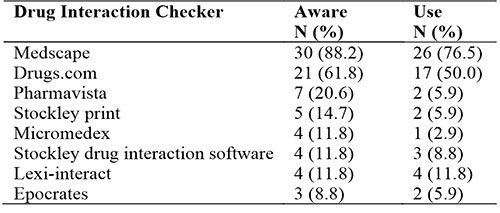
Table 3: Association between awareness of drug-drug interaction checkers to age and rank
Regarding the use of drug-drug interaction checkers, 26 (76.5%) residents used Medscape followed by 17 (50%) who said to have used drugs.com. The lowest interaction checker used was Micromedex with 1 (2.9%) respondent. A respondent found Medscape and drugs.com easy to use while Pharmavista was not easily assessable. Also, drugs.com was not inclusive of some medications. Another respondent found Medscape to be internet-dependent. However, a respondent found drugs.com and Medscape easy to use and enlightening. A resident found Lexi-interact to be clear and concise. Table 3 showed that there was no statistical significance between the awareness of drug-drug interaction checkers to age and rank of internal medicine residents in the University of Benin Teaching Hospital.
Discussion
Our study on awareness of resident doctors and use of drug interaction checkers was high for Medscape {30 (82.2%)} and low for Epocrates {3 (8.8%)}. Regarding the use of drug-drug interaction checkers, 26 (76.5%) residents used Medscape followed by 17 (50%) recorded to have used drugs.com. The lowest interaction checker used was Micromedex 1(2.9%). A respondent found Medscape and drugs.com easy to use while Pharmavista was not easily assessable. Ease of use and accessibility appeared to be key issues encouraging the use of the interaction checkers. This study also showed that there was no statistical significance between the awareness of drug-drug interaction checkers and to age and rank of medical residents which include registrars and senior registrars. A study on the knowledge and attitude of community pharmacists showed, most got their sources of drug interactions from online applications such as Medscape, drugs.com, e-Library platform, Micromedex, and Google while others consulted their colleagues, or books or by a phone call to a drug representative9 In Iran, sources of drug-drug interaction information among physicians revealed that 42.7% assessed a book,33.5% used software on mobile or tablet while 15.3% used internet sources.10 Yuan et al in a study showed that the use of package inserts was 92.6% while the less frequently used tools were internet or mobile Apps (68.6%), textbooks (60.5%), and other sources were consultations with physicians and pharmacists.11 A United States study revealed that prescribers used personal digital assistants (PDA) (25.9%) while the use of printed materials was 24.1%. Approximately 14.2% of prescribers used both package inserts and pharmacists as their sources.12 In another study among health workers in a Malaysian general hospital, it was observed that most of the respondents used computerized interaction checkers (76.4%), and had many educational programs on drug interactions (75.5%).13 Research in China showed that the most popular information source utilized by doctors to assess PDDIs was the package insert, which is a significant risk factor for the inaccurate use of medication. Electronic databases or computerized systems are scarce in China; it would be challenging for doctors to be up to date with recent evidence due to their hectic schedules and they may not be able to check package inserts for every suspected drug interaction, which poses serious harm to patient safety. Consequently, readily available scientific resources should be accessible to doctors to increase drug safety. Even with the aid of automated PDDI detection devices, prevention of DDIs is extremely challenging, especially as multiple medicines are prescribed to patients routinely.11
A study of a group of 119 resident doctors in UBTH found the monthly index of medical specialties as the most frequently used source of drug information and drug-drug interactions was about 85% of the information sought.7 Another study by Olowofela et al14 found that among 198 residents, the preferred site for searching for drug-drug interaction was in the following order: Google, Medscape, and Wikipedia.14
Computerized physician order entry systems make available process development, improved order clarity and correctness, and integration of clinical decision support assistance with order entry, time management for doctors, nurses, and pharmacists, drug allergy checks, and detection of drug interactions and improper dosages.15 Alert systems that are more well-liked are those that offer both high-quality information and user-friendly message design, including in-depth guidance.16 Novice prescribers could be hesitant to alter existing prescription orders, especially in the lack of suggestions for alternative medications while senior clinicians can disagree with the clinical importance of the reported drug-drug interactions because patient factors and circumstances were not taken into account by the drug-drug interaction checker,16 Morrell et al found drug-drug interactions reports were sent to prescribers using an automated computer service. Due to their inexperience, the authors claimed that medical students and interns could benefit the most from the service.17 To increase patient safety, many computerized physician order entry systems have integrated drug safety alerts. However, overriding drug safety alarms has received scant attention from researchers.18
Limitation: This study was carried out in the internal medicine unit of the University of Benin Teaching Hospital, Benin City and this limited the sample size.
Conclusion
This study highlights the utility of interaction checkers in detecting potential drug-drug interactions suggesting a significant clinical application in preventing harm.
Nevertheless, there is adequate awareness and use of Medscape amongst clinicians but low awareness for Epocrates and poor use of Micromedex
Ethics approval and consent to participate: The University of Benin Teaching Hospital Research Ethical Committee gave its approval to the study. ADM/E 22/A/VOL VII/14830945, is the protocol number, and consent was obtained from physicians. Study participants were required to provide informed consent voluntarily and with full knowledge of the study's objectives. All methods were used in compliance with all applicable rules and regulations.
Competing interests: No conflict of interest
Funding: Self-Funded
Authors' contributions: OOG, OAO, IAO wrote the manuscript, OOG, OAO, IAO designed the study, OOG analyzed the data, OOG performed research OOG contributed to the analytical tools.
References
- Baxter K. General considerations and an outline survey of some basic interaction mechanisms. In: Stockley’s drug interactions. 9th ed. London: Pharmaceutical Press; 2010. p. 1–11.
- McInnes GT, Brodie MJ. Drug Interactions that Matter: A Critical Reappraisal. Drugs. 1988;36:83–110.
- Weingart SN. An Empirical Model to Estimate the Potential Impact of Medication Safety Alerts on Patient Safety, Health Care Utilization, and Cost in Ambulatory Care. Arch Intern Med. 2009;169:1465–73.
- Ko Y, Abarca J, Malone D, Dare D, Geraets D, Houranieh A, et al. Practitioners’ views on computerized drug-drug interaction alerts in the VA system. J Am Med Inform Assoc. 2007;14:56–64.
- Ko Y, Malone D, Skrepnek G, Armstrong E, Murphy J, Abarca J, et al. Prescribers’ knowledge of and sources of information for potential drug-drug interactions: a postal survey of US prescribers. Drug Saf. 2008;31:525–36.
- Lancet T. Rational use of medicines. The Lancet. 2010;375:2052.
- Olowofela A, Ayinbuomwan S, Isah .A.O. Sources of information on the use of medicines utilized by resident doctors in a tertiary health care facility in Nigeria. Highland Med Res J. 2017;17(2):81-85.
- Moura CS, Prado NM, Belo NO, Acurcio FA. Evaluation of drug–drug interaction screening software combined with pharmacist intervention. Int J Clin Pharm. 2012;547–52.
- Makkaoui N, Halaoui A, Atoui Z, Siblini H, Habib S, Awada H, et al. Knowledge, attitudes, and practices regarding drug interactions among community pharmacists. J Public Health (Berl). 2020;1357–63.
- Nabovati E. A survey of attitudes, practices, and knowledge regarding drug–drug interactions among medical residents in Iran. Int J Clin Pharm. 2017;560–8.
- Yuan J, Shen C, Wang C, Shen G, Han B. Assessment of Physician’s Knowledge of Potential Drug-Drug Interactions: An Online Survey in China. Front Med. 2021;8:650369.
- Ko Y, Malone DC, Skrepnek GH, Armstrong EP, Murphy JE, Abarca J, et al. Potential determinants of prescribers’ drug-drug interaction knowledge. Drug Saf. 2008;31:525–36.
- Abdo MS, Hammad MA, Harun SN, Eusoff TM, Sheikh Ghadzi SM. Evaluation of knowledge, attitude and practice of healthcare providers towards life-threatening drug-drug interactions in Penang General Hospital, Malaysia. Clin Epidemiology Glob Health. 2020;8:1253–8.
- Olowofela A, Ayinbuomwan S, Isah .A.O. The internet as a source of drug information:a profile of utilization by junior hospital doctors in a Nigerian teaching hospital. Ann Biomed Sci. 2017;16:226–33.
- Oren E, Shaffer ER, Guglielmo BJ. Impact of emerging technologies on medication errors and adverse drug events. Am J Health Syst Pharm. 2003;60:1447–58.
- Beeler PE, Eschmann E, Rosen C, Blaser J. Use of an On-demand Drug–Drug Interaction Checker by Prescribers and Consultants: A Retrospective Analysis in a Swiss Teaching Hospital. Drug Saf. 2013;36:427–34.
- Morrell J, Podlone M, Cohen S. Receptivity of physicians in a teaching hospital to computerized drug interaction monitoring and reporting system. Med Care. 1977;15:68–78.
- Heleen V.d. S, Jos Aarts, A.V, Marc B. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13(2):138–47.