Ileostomies for severely ill children with ileo-colic intussusception: our experience at the University of Uyo Teaching Hospital
Akpanudo EI, Ituen AM, Eyo AE, Emmanuel EM
Abstract
Context: Complicated intussusception is associated with life-threatening intestinal gangrene and peritonitis. In critically ill children, a primary ileocolic anastomosis may increase their morbidity and mortality and, thus, a temporary ileostomy may be a safer option.
Objective: The purpose of this study is to report the indications, outcomes, and complications after temporary ileostomies for infants with ileocolic intussusception in our institution.
Materials and methods: This was a retrospective study of infants who had ileostomies created in our institution following complicated ileocolic intussusceptions between January 2014 and December 2022. The indications, postoperative complications, duration of hospital stay, and recovery were noted and compared.
Results: Thirty-four infants had ileostomies created for complicated ileocolic intussusception during this period. There were 13 girls and 21 boys aged 3-10 months (median = 6 months). The indications for ileostomy were intestinal gangrene, colonic perforation, peritonitis, and haemodynamic instability. Ileostomies were created in the primary surgery in 24 patients while 10 had stomas created as a secondary procedure following an anastomotic dehiscence. Postoperative complications occurred in 25 patients (73.5%). Four children died shortly after surgery from the primary disease, while one child died from re-feeding syndrome. Children who required stoma creation following dehiscence of a primary anastomosis had more complications, a longer hospital stay, and a longer delay in commencing feeds. However, there was no difference in mortality rates or time of stoma closure.
Conclusion: Temporary ileostomies could potentially reduce intussusception-related mortality and morbidity, ultimately improving the outcome of very ill infants.
Keywords: Temporary ileostomy, intussusception, anastomotic dehiscence, intestinal gangrene, peritonitis.
Introduction
Intussusception is the invagination of a proximal bowel segment into an adjoining distal segment. It is the most common cause of intestinal obstruction in children less than one year of age in our sub-region.1-3 The ileocolic variant, where the ileum telescopes through the ileocaecal valve into the colon, is the commonest, accounting for about 78% of cases.4,5
Early diagnosis, aggressive resuscitation, and rapid reduction are essential for the successful management of these children. Preferred treatment options for intussusception include non-operative (pneumatic or hydrostatic) enema reduction or operative manual reduction when non-operative options fail. However, patients in our environment frequently present late with intestinal gangrene, perforation, and peritonitis. The treatment of these complicated cases is invariably surgical, involving small and large bowel resections, followed by either a primary ileo-colic anastomosis or exteriorization.6-8
When primary ileocolic anastomoses are deemed unsafe, temporary ileostomies are considered to be life-saving procedures. A temporary stoma may be beneficial for the patient in situations where there is a high risk for an anastomotic leak (such as in patients who are hemodynamically unstable, have peritonitis, are malnourished, etc.).9-13 Despite this, creating a temporary ileostomy is rarely done as a primary treatment option for intussusception in our environment. One can only speculate that many surgeons may be reluctant due to the anticipated stoma-related complications and impact on the patient’s postoperative quality of life.
The purpose of this study is to report our experience with ileostomies created for critically ill infants with intussusception, highlighting the common indications, the post-operative complications, and outcomes, as well as making recommendations for our regional practice.
Materials and methods
This was a retrospective descriptive study conducted by the Paediatric Surgery Unit of the University of Uyo Teaching Hospital, Uyo, Southern Nigeria, from January 2014 to December 2022. All paediatric patients managed for intussusception, who had temporary ileostomies created during this nine-year period were included in this analysis. We made a clinical diagnosis of intussusception for all patients with a detailed history, physical examination, and abdominal ultrasound. The patients were resuscitated and had exploratory laparotomies. The nature of peritoneal fluid, length of bowel involvement, and nature of the bowel walls were noted. Ileostomies were fashioned for patients who had ileocolic gangrene, colonic perforations, severe peritonitis with oedematous bowel, were haemodynamically unstable (defined as having a systolic blood pressure of less than 70 mmHg and a capillary refill time of more than 3 seconds), and required extensive resections. These were either divided ileo-colostomies or end ileostomies. Following surgery, the infants were started on oral feeding within 48–72 hours if tolerated and in the absence of ileus. Their serum electrolytes were measured at least twice during the first week. After discharge, the infants were followed up in the outpatient clinic to monitor for late complications and recovery from surgery. Stomas were scheduled for reversal when the patients were considered fit.
Data collected included patients’ ages at the time of presentation, gender, duration of symptoms before surgery, reasons for delayed presentations, intraoperative findings, the time of commencement of oral feeding, duration of hospital stay, post-operative complications (occurring during their hospital stay and subsequent follow-up in the outpatient clinic), mortalities, and time of stoma reversal.
The statistical analysis for this study was done with SPSS Statistics 23 for Windows.14 The results of the univariate analyses were described in terms of ranges and medians (plus interquartile ranges [IQR]) for continuous variables. Categorical variables were described in terms of frequencies and percentages. The statistical significance of the data was analysed using the Mann-Whitney-U test or the Fisher Exact test. P-values and effect sizes (r) were presented. The Bonferroni correction was applied to account for multiple comparisons. For this analysis, after Bonferroni adjustment, a p value < 0.007 was considered statistically significant.
Results
During the study period, 164 patients presented with intussusception to our institution. One hundred and thirty-eight (84.1%) of these patients required exploratory laparotomy for intussusception. Of this number, 34 (24.6%) required a temporary ileostomy. There were 21 males and 13 females. Their ages ranged from 3 to 10 months (median = 6, IQR = 4 – 6). The duration of symptoms before presentation to our unit ranged from 8 to 23 days (median = 14.5, IQR = 10 – 15). The pattern of presentation is summarised in Figure 1 and Table 1.
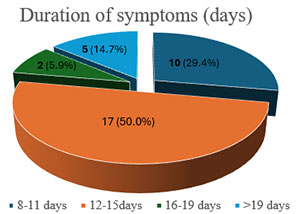
Figure 1: Duration of Symptoms
Table 1: Reasons for delayed presentation
All patients had extensive ileo-colic gangrene and/or perforation with peritonitis. Twenty-four (24) patients (70.6%) had primary stomas created, while 10 patients (29.4%) required stomas after postoperative dehiscence of an ileo-colic anastomosis. The intraoperative findings/factors determining stoma creation are summarised in table 2.
Table 2: Intraoperative Findings
Five (14.7%) of the patients died: 4 severely ill patients died within the first 24 hours after surgery, while 1 patient died within the first week after stoma creation from refeeding syndrome.
Four patients (11.8%) had no complications on discharge, while 25 patients (73.5%) developed complications. Peri-stomal skin excoriation or dermatitis was the most common complication, occurring in 24 (70.6%) of the patients. Infections of the main laparotomy wound occurred in 12 patients (35.3%), while electrolyte anomalies were noticed during the first post-operative week in 8 patients (23.5%). The postoperative complications are summarised in Table 3.
Table 3: Post-operative Complications for 34 patients
The length of hospital stay for these patients ranged from 7 to 60 days. The median duration of hospital stay was 10 days (IQR = 8-14) for patients who had primary ileostomies, while the median was 21 (IQR = 19-48) days for those who had secondary ileostomies. Stomas were reversed between 5 and 32 weeks postoperatively (median = 16, IQR = 12 – 20.5).
The patients who required stoma creation following dehiscence of a primary anastomosis had a twofold greater number of complications, a twofold longer duration of hospital stay, and a twofold longer delay in commencing feeds; however, there was no significant difference between mortality or the duration until stoma reversal in both groups (Table 4).
Table 4: Comparison of Patients who had Primary or Secondary Ileostomies
Discussion
The negative consequences of sepsis and inflammation are well documented in critical care surgery. Treatment of intraabdominal sepsis (IAS) can be challenging and associated with high mortality rates.15 One method of mitigating the negative effects of sepsis and inflammation is Damage Control Surgery, which is based on the principles of temporal control of the illness or injury with the inclination to restore normal physiology over normal anatomy in the critically ill, unstable patient. Ameh et al.16 Described the creation of temporary enterostomies as a damage control measure in infants and children with severe abdominal disease and peritonitis, as the decision to perform an intestinal anastomosis in the setting of an ill patient with intrabdominal sepsis can be risky. To the best of our knowledge, there is limited publication on the formation of temporary ileostomies for infants with intussusception in our subregion.
The rate of temporary ileostomies created for intussusception patients in our center was 24.6%, which is higher than the rates reported by Chalya et al.17 (3.6%) and Kuremu18 (8%). Ameh et al6 also reported that fewer than 10% of their children required ileostomies, which were all offered as salvage procedures following anastomotic dehiscence. Our observations may not be unrelated to the prolonged duration of symptoms prior to presentation (8-23 days) that was common among our patients and which, in our opinion, contributed to the severity of their illness. This is longer than noted in other studies from our region, which indicate that patients with intussusception typically present within three to five days.5,6,8,17-19 However, a few have also noted delays of up to two to three weeks4,17,19 Late presentation contributes significantly to the high morbidity and mortality rates associated with intussusception and other intestinal disorders due to its association with worsening patient conditions and increased tendency towards devitalized bowel and intestinal resections.4-8,17,19-24 In our setting, late presentations are commonly attributed to poverty and ignorance; however, we have found that delayed referrals are often due to misdiagnosis.8,20,21 Several authors have also observed that many children are initially managed in peripheral hospitals, with referral to specialist centres only after their clinical conditions have deteriorated considerably.4,8,17-19 Considering that the patients who presented late had poorer general conditions, we often opted for primary ileostomies for children whose symptoms were longer than 7 days, with better outcomes. This approach aligns with findings from other studies, which also noted the advantage of exteriorization over primary intestinal anastomosis in patients who present late.9,11
In severe peritonitis, the risk of anastomotic dehiscence is high due to various systemic and local factors affecting healing.9,25,26 Thus, in addition to the duration of symptoms, the decision to create stomas in our study was based on specific conditions often observed in patients with peritonitis. These include the presence of haemodynamic instability, faeculent and/or purulent intraperitoneal fluid, and oedematous bowel ends. In addition, patients who required bowel resection extending to the distal colon or who had anastomotic dehiscence were also considered for ileostomy creation. Ameh et al.16 used similar criteria in determining children who required enterostomies, emphasising the importance of these factors in wound healing and overall patient outcomes. Though it could be argued that adequate preoperative resuscitation could ameliorate the haemodynamic instability, it is, however, difficult to resuscitate a patient with diffuse peritonitis from a perforated viscus until the ongoing soilage is controlled.25 Weledji et al.25 and Walbel et al.26 stressed the importance of offering such patients resuscitative (damage control) surgery with temporary stomas. Similarly, for resections up to the distal sigmoid colon in our very ill patients, we decided to create ileostomies because, as Snyder et al.27 explained, there is a greater risk of anastomotic failure, related to the tenuous blood supply of the sigmoid colon, especially in times of increased vascular need. The authors further explained that the risk of anastomotic leakage in the distal colon increases with hypoalbuminaemia, prolonged operating time (>200 minutes), the need for intraoperative blood transfusions, and the presence of infections.
Complications from surgery in these patients have significant morbidity and are a frequent cause of distress for both patients and caregivers. Some complications were due to the severity of the primary illness, while others were related to the ileostomies. The complication rate in our study was 73.5%. This is similar to results found in other studies by Miyo et al.28 and Mehboob et al.29, who recorded complication rates of 83% and 72.6%, respectively; however, it is important to note that our sample size was smaller in comparison to these studies.
Noted complications included:
Peristomal Skin Excoriation: Peristomal skin excoriation was the commonest early complication seen in our patients, occurring in 70.6%. Other studies also agreed that skin excoriation was the most frequent ileostomy related complication16,26-30. We managed this by educating parents early on the importance of applying barrier ointments (petroleum jelly and zinc oxide cream) generously to the peristomal skin.
Electrolyte abnormalities: With the exteriorization of the small bowel, it is expected that the absorption of water, proteins, carbohydrates, lipids, Vitamin B12, and electrolytes viz sodium, potassium, and magnesium will be negatively impacted, exposing these patients to dehydration, electrolyte imbalances, and malnutrition. Interestingly, serum electrolyte abnormalities occurred in 23.5% of patients in our study but were not the most notable cause of postoperative morbidity, as they were transient. We ensured that our postoperative intravenous fluids were isotonic, and potassium was added to the intravenous fluids at maintenance levels. In addition, parents were advised to add moderate amounts of salt to their feed and to increase water intake in the first few weeks. When the intake of sodium, potassium, and fluids is adequate, patients with terminal ileum ileostomies do not experience significant electrolyte depletion30. Ameh et al16 observed that 8% of their patients died from enterostomy related electrolyte abnormalities. However, their study reported enterostomies created for various pathologies, involving different segments of the ileum. Therefore, it is possible that some stomas in that series were created using more proximal parts of the ileum, which are associated with higher outputs and higher fluid and electrolyte losses. Our patients all had ileostomies created using the terminal ileum. Studies have suggested that ileostomies created without significant ileal resection did not have significant effects on body fluid and electrolyte composition29-35.
Stoma diarrhoea: All the ileostomies created in this report began to function within 24-48 hours. The stools were initially watery and had large volumes, but this typically decreased gradually between the second and sixth weeks due to ileostomy adaptation. Despite this compensation, there is a considerable risk of severe dehydration in the immediate postoperative period if oral or intravenous fluid intake is inadequate. Ileostomy diarrhoea, or high-output syndrome, is a dreaded complication of ileostomy surgery that typically develops during the first 15 days of the procedure and causes protein and electrolyte loss in addition to fluid loss. The length of the ileum that is removed also influences the degree of adaptation. Our ileostomy diarrhoea complication rate was low because the ileostomies in our study were fashioned close to the terminal ileum, which contributed to their moderate output (8.8%). This was comparable to rates reported by Mehboob et al.29 (3.5%), but lower than reported by Takeda et al.36 (23.8%) and Justiniano et al.37 (26%).
Protein energy malnutrition and refeeding syndrome: Seven patients (20.6%) in our study developed Protein-energy malnutrition in the post-operative period, requiring referral to paediatricians and nutrition specialists. They had lost more than 10–15% of their admission weight at the time of discharge or within the first 6 weeks after surgery, despite our efforts to ensure adequate nutrition and fluid intake. Mohil et al.38 also studied the impact of ileostomies on the nutritional status of 75 patients. In their series, 70% of their patients had severe weight loss, which they attributed to the length of small bowel left, the ileostomy output, and the severity of the primary disease. Early return to feedings is an important factor in preventing malnutrition in these patients, as studies have shown that early postoperative feeding and adequate nutritional support can reduce the catabolic response to surgery and shorten recovery time.39-44 We were able to start oral intake within 48–72 hours for most of our patients (67.6%), which may have contributed to the early recovery and overall favourable outcomes of most of our patients. However, the desire to feed these children early and aggressively must be weighed with their risk for refeeding syndrome. Refeeding syndrome is a potentially fatal metabolic disorder that occurs on reintroduction of enteral or parenteral nutrition after prolonged starvation or reduced energy intake.45,46 Infants at risk of developing refeeding syndrome are those who have had reduced energy intake for 7–10 days before reintroduction of feeds. Most cases are recorded as occurring within the first 1–3 days after refeeding is started. One child in our series was clinically diagnosed to have developed refeeding syndrome and eventually died on the third day of refeeding. Recommendations to prevent refeeding syndrome include cautious feeding with slow graded increments over several days. In accordance with WHO and ESPNIC recommendations, we recently started feeding our patients at a caloric level equal to the child's REE calculated using the Schofield equation.47-49
Poor wound healing and wound related complications (surgical site infection, wound dehiscence, incisional hernias, and stomal detachment): In our study, 22 wound related complications were recorded. We found the incidence of surgical site infection was 35.3% (12/34), wound dehiscence was 14.7% (5/34) and incisional hernia was 11.8% (4/34). This is similar to other reports, which have incidences of wound related complications after emergency abdominal surgery ranging from 21-26%12,29,50-53. Factors identified in the literature to be associated with suboptimal wound healing include very young age (> 1 year), late presentation, inanition, the need for emergency surgery, intrabdominal sepsis, and postoperative ileus.52,53 We noted that pre-operative malnutrition due to late presentations was an important factor affecting the postoperative recovery of these patients. Wound outcomes in children are related to the degree of preoperative hypoproteinemia54.
Prolonged hospital stay: We observed an overall median duration of stay of 14 days for our patients. This was primarily due to the need to treat wound-related complications and ensure that the parents or caregivers were taught how to manage the ileostomies at home. The overall duration of the postoperative hospital stay was not longer than what other surgeons who managed complicated intussusceptions reported. Bode4 reported the mean duration of hospital stay in Lagos, Nigeria, to be 17 days, while Ogundoyin et al.8 noted a mean hospital stay of 12.1 days, and Chalya et al.17 reported a median stay of 14 days in their patients. Our results show that creating ileostomies does not necessarily increase the duration of hospital stay when compared to other children with complicated intussusception. However, it is important to note that the duration of hospital stay was significantly longer in our patients who had ileostomies created as secondary procedures (median = 21 days) than in those who had primary ileostomies (median = 10 days) (p<0.001). This suggests that ileostomies created in a timely manner would not only benefit the patients by improving their overall recovery but also potentially reduce their length of hospital stay. However, a larger comparative study would be necessary to further validate these findings.
Reports of mortality associated with ileostomy creation are low in most literature. Rates found ranged from 46%16 to as low as 1.79%,31 to none.29 Most studies reported that the mortalities were related to the severity of the primary disease, while few were associated with the stoma. Common risk factors associated with deaths in these children were reported as delayed presentation, very young age, peritonitis, sepsis, electrolyte abnormalities, and abdominal re-exploration.16,31 We reported 5 deaths over the study period, with an overall mortality rate of 13.9%. Four patients who were severely ill died within the first 24 hours after surgery, while one patient died within the first week from refeeding syndrome. The ages of these children were 3 months in one child, 4 months in three children, and 6 months in the final child. In addition to their young age, three of these children had ileostomies created on re-exploration, after a failed primary anastomosis.
The ileostomies in this study were reversed between 5 and 32 weeks. There was no statistically significant difference in the stoma closure time between the two groups (p = 0.564). Other studies showed similar results, with stomas typically reversed between 6 and 40 weeks.16,18 Some of the delays in stoma reversal in our setting were due to patient-related factors like prolonged malnutrition; however, there were many institutional and system-related factors that also contributed to the delays. These include the long surgical wait times for elective surgery in our institution, scheduling conflicts, and the prohibitive cost of additional surgery for many families. These findings suggest that the reversal of a protective ileostomy is unaffected by the primary indication for the surgery, and that it can be successfully performed at various intervals.
Limitations
This study was not without limitations. First, it has been noted that biases are more frequent in data collected retrospectively.55, 56 Second, there is the possibility that type 1 errors, due to the relatively smaller number of subjects in the secondary ileostomy group, may have contributed to the larger difference in stoma-related complications between the two groups. Further research with larger sample sizes is needed to determine if the disparity in stoma-related complications between the two groups is statistically significant.
Conclusion
In this study, we looked at the indications for temporary ileostomies and the post-operative morbidity in infants for whom they were created. We found that the procedure was safe, manageable, and effective in treating intussusception in some infants. The indications for creating temporary ileostomies were mainly to relieve bowel obstruction, offer faecal diversion, and prevent further complications related to peritonitis and sepsis. Though stoma related complications were noted, most infants recovered well and experienced no long-term complications. Overall, our report showed that temporary ileostomies are a viable option for the management of intussusception in infants, providing a safe and lifesaving treatment option for very ill patients in our resource-poor environment. We recommend that a temporary ileostomy be considered for very ill children with intestinal gangrene, perforation, and peritonitis, or whenever an anastomotic leakage is suspected in the postoperative period. Limiting the risk of further peritoneal contamination can reduce mortality in these patients. To provide more comprehensive data for decision making, we also recommended that a larger cohort of patients be studied to further compare the outcomes of primary anastomosis with temporary ileostomies.
References
- Uba AF, Edino ST, Yakubu, A, and Sheshe, AA. Childhood intestinal obstruction in northwestern Nigeria. West African Journal of Medicine, 2004: 23(4): 314–318
- Ogundoyin OO, Afolabi AO, Ogunlana DI, Lawal TA, and Yifieyeh A. Pattern and outcome of childhood intestinal obstruction at a tertiary hospital in Nigeria. African Health Sciences. 2009; 9(3): 170–173.
- Ooko PB, Wambua P, Oloo M, Odera A, Topazian H, White R. The spectrum of paediatric intestinal obstruction in Kenya. Pan African Medical Journal, 2016; 24(43)
- Bode CO. Presentation and management outcomes of childhood intussusception in Lagos: a prospective study. African Journal of Paediatric Surgery, 2008; 5(1): 24-28
- Ekenze SO, Mgbor SO. Childhood intussusception: the implications of delayed presentation. African Journal of Paediatric Surgery, 2011; 8(1): 15–18
- Ameh EA. The morbidity and morbidity of right hemicolectomy for complicated intussusception in infants. The Nigerian Postgraduate Medical Journal, 2002; 9(3): 123–124
- Ekenze SO, Mgbor SO, Okwesili OR. Routine surgical intervention for childhood intussusception in a developing country. Annals of African Medicine, 2010; 9(1): 27–30.
- Ogundoyin OO, Olulanla DI, Lawal TA. Childhood intussusception: impact of delay in presentation in a developing country. African Journal of Paediatric Surgery, 2016; 13(4): 166–169
- Rasslan S, Fonoff AM, Solda SC, Casaroli AA. Ostomy or intestinal anastomosis in cases of peritonitis. Sao Paulo Medical Journal/RPM, 1995; 113(6):1017–1021.
- Ordóñez CA, Sánchez AI, Pineda JA, Badiel M, Mesa R, Cardona U, et al. Deferred primary anastomosis versus diversion in patients with severe secondary peritonitis managed with staged laparotomies. World Journal of Surgery, 2010; 34(1): 169–176
- Pipariya PJ, Menon AV, Chandel H. A comparative study of primary repair vs. stoma in emergency surgeries: an institutional experience. Scholars Journal of Applied Medical Sciences, 2015; 3(31): 1326–1331
- Nadeem M, Bashir MM, Iqbal J, Rasheed A. Primary repair vs. colostomy for colonic injuries. AM KE MED COLL. 2004; 10: 462–5.
- Alhamdani AK, Albadri JM, Abed HJ, Abed HJ. Primary repair vs. diversion in penetrating colonic injuries. Al-Kindy Col Med J. 2013; 9(1); 71–79
- IBM Corp. (2015). IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.
- Faes S, Hübner M, Girardin T, Demartines N, Hahnloser D. Rate of stoma formation following damage-control surgery for severe intra-abdominal sepsis: a single-centre consecutive case series. BJS Open, 2021 Nov 9;5(6)
- Ameh E, Ayeni M, Kache S, Mshelbwala P. Role of damage control enterostomy in management of children with peritonitis from acute intestinal disease. African Journal of Paediatric Surgery, 2013; 10(4): 315–319
- Chalya P, Kayange N, Chandika A. Childhood intussusceptions at a tertiary care hospital in Northwestern Tanzania: a diagnostic and therapeutic challenge in resource-limited setting. Italian Journal of Pediatrics, 2014; 40:28.
- Kuremu RT. Childhood intussusception at the Moi Teaching and Reference Hospital, Eldoret: management challenges in a rural setting. East African Medical Journal, 2004; 81: 443–44
- Ogundoyin OO, Olulana DI, Lawal TA. Childhood intussusception: a prospective study of management trends in developing countries. African Journal of Paediatric Surgery, 2015; 12(4): 217–220
- World Health Organization, Vaccines and Biologicals. Acute intussusception in infants and children. Incidence, clinical presentation, and management: a global perspective. 2002, Geneva:WHO
- Hsiao HJ, Wang CJ, Lee CC, Hsin YC. Point-of-care ultrasound may reduce misdiagnosis of pediatric intussusception, Frontiers in Pediatrics, 2021; 9:601492.
- Oleribe OO, Ezieme IP, Oladipo O, Akinola EP, Udofia D, Taylor-Robinson SD. Industrial action by healthcare workers in Nigeria in 2013–2015: an inquiry into causes, consequences, and control—a cross-sectional descriptive study. Human Resources for Health, 2016; 14:46
- Hazra NK, Karki OB, Verma M, Rijal D, De A, Nath B. Intussusception in children: a short-term analysis in a tertiary care hospital. American Journal of Public Health Research. 2015; 3(4A): 53–56
- Adejuyigbe O, Jeje EA, Owa JA. Childhood intussusception in Ile-Ife, Nigeria. Annals of Tropical Paediatrics, 1991; 11(2): 123-127
- Weledji EP, Ngowe MN. The challenge of intra-abdominal sepsis. International Journal of Surgery. 2013; 11(4): 290–295.
- Walbel B, Rotundo MF. Damage control for intrabdominal sepsis. Surgical Clinics of North America. 2012; 92(2): 243-257.
- Snyder J, Croce M. Management of anastomotic leaks – early <7 days and late >7 days. In: Diaz J, Effron D., editors. Complications in Acute Care Surgery. The Management of Difficult Clinical Scenarios. Cham: Springer International Publishing, 2017.
- Miyo M, Takemasa I, Ikeda M. The influence of specific technical maneuvers utilized in the creation of diverting loop-ileostomies on stoma-related morbidity, Surgery Today 2017:47: 940-950
- Mehboob A, Perveen S, Iqbal M, Bux KM, Waheed A, Frequency and complications of ileostomy, Cureus 2020 12(10):e11249
- Aziz A, Sheik I, Jawaid M, Alam SN, Saleem M. Indications and complications of loop ileostomy. Journal of Surgery Pakistan (International). 2009; 14(3): 128–131.
- Ghritlaharey RK, Budhwani KS, Shrivastava DK. Exploratory laparotomy for acute intestinal conditions in children: A review of 10 years of experience with 334 cases. African Journal of Paediatric Surgery 2011; 8(1): 62–69
- Chaudhary P, Nabi I, Ranjan G, Tiwari AK, Kumar S, Kapur A, et al. Prospective analysis of indications and early complications of emergency temporary loop ileostomies for perforation peritonitis. Annals of Gastroenterology, 2015; 28:135–140.
- Cooper JC, Laughland A, Gunning EJ, Burkinshaw L, Williams NS. Body composition in ileostomy patients with and without ileal resection. Gut. 1986; 27(6):680–685.
- Cima RR, Pemberton JH. Ileostomy, colostomy, and pouches. In: Feldman M, Friedman L, Brandt L., editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Diseases. 9th edition. Philadelphia:Saunder 2010
- Ganguly SS, Saha AK, Jha S, Bhattacharyya S, Kuiri SS, Das C. Evaluation of change in serum sodium and potassium following ileostomy and colostomy. IOSR Journal of Dental and Medical Sciences. 2014; 13(7) Ver II: 38–46
- Takeda M, Takahashi H, Haraguchi N, Miyoshi N, Hata T, Yamamoto H, et al. Factors predictive of high-output ileostomy: a retrospective single-center comparative study. Surg Today. 2019 Jun; 49(6):482-487. doi: 10.1007/s00595-018-1756-2.
- Justiniano CF, Temple LK, Swanger AA, Xu Z, Speranza JR, Cellini C, et al. Readmissions With Dehydration After Ileostomy Creation: Rethinking Risk Factors. Diseases of the Colon and Rectum. 2018 Nov;61(11):1297-1305.
- Mohil RS, Narayan N, Sreenivas S, Namrata S, Abhinav B, Gulshan JS, Challenges of Managing Emergency Ileostomy: Nutritition – A Neglected Aspect. International Scholarly Research Notices, vol. 2012, Article ID 968023
- Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013 Sep;37(5 Suppl):21S-9S. doi: 10.1177/0148607113496117.
- Correia MI, da Silva RG. The impact of early nutrition on metabolic response and postoperative ileus. Curr Opin Clin Nutr Metab Care. 2004 Sep;7(5):577-83. doi: 10.1097/00075197-200409000-00011. PMID: 15295279.
- Sholadoye T, Suleiman A, Mshelbwala P, Ameh E. Early oral feeding following intestinal anastomoses in children is safe. African Journal of Paediatirc Surgery. 2012; 9(2): 113-116
- Amanollahi O, Azizi B. The comparative study of the outcomes of early and late oral feeding in intestinal anastomosis surgeries in children. African Journal of Paediatric Surgery. 2013; 10(2): 74–77
- Mamatha B, Alladi A. Early oral feeding for pediatric intestinal anastomosis. Indian Journal of Surgery. 2015; 77(Supl 2):670-672.
- Lu C, Sun X, Geng Q, Tang W. Early oral feeding following intestinal anastomosis surgery in infants: A multicenter real-world study. Frontiers in Nutrition 2023, Vol 10, doi: 10.3389/fnut.2023.1185876.
- Marino LV, Chaparro CJ, Moullet C, Re-feeding syndrome and other related issues in the paediatric intensive care unit, Pediatric Medicine 2020, Vol 3.
- Blanc S, Vasileva T, Tume L, Baudin F, Ford C, Jotterand C, et al. Incidence of refeeding syndrome in critically ill children with nutritional support. Frontiers in Pediatrics 2022, doi: 10.3389/fped.2022.932290
- Schofield WN, Predicting basal metabolic rate, new standards, and review of previous work. Human Nutrition: Clinical Nutrition. 1985, 39 Suppl 1:5–41
- World Health Organisation, FAO, and UNU. Energy and protein requirements. Geneva: WHO, Technical Report Series 724, 1985.
- Tume LN, Valla FV, Joosten K, Jotterand CC, Latten L, Marino LV, et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine, and nutrition section position statements and clinical recommendations. Intensive Care Medicine 2020, 46(3): 411-425
- Ameh EA, Mshelbwala PM, Nasir AA, Lukong CS, Jabo BA, AnumahMA, et al. Surgical site infections in Children: prospective analysis of the burden and risk factors in a sub-Saharan African setting, Surgical Infections 2009, 10(2), pp. 105–109.
- Chukwubuike KE. Postoperative wound complications following abdominal surgery in children: A single centre experience. Archives of Clinical and Experimental Surgery, 2021, 10(9), pp. 01–05
- Osifo OD, Ovueni ME. The predictors, prevalence, and outcome of burst abdomen in an emergency paediatric surgical centre. East and Central African Journal of Surgery, 2010, 15(2)
- van Ramshorst GH, Salu NE, Bax NM, Hop WC, van Heurn E, Aronson DC, et al. Risk factors for abdominal wound dehiscence in children: A case-control study, World Journal of Surgery 2009 33(7): 1509–1513
- Duan S, Zhang X, Jiang X, Ou W, Fu M, Chen K, et al. Risk factors and predictive model for abdominal wound dehiscence in neonates: a retrospective cohort study. Ann Med. 2021 Dec;53(1):900-907. doi: 10.1080/07853890.2021.1938661.
- Shafer SL, Dexter F. Publication Bias, Retrospective Bias, and Reproducibility of Significant Results in Observational Studies. Anesthesia & Analgesia 2012, 114(5): pp. 931–932.
- Sauerland S, Lefering R, Neugebauer EA. Retrospective clinical studies in surgery: potentials and pitfalls. J Hand Surg Br. 2002; 27(2):117–21.