Aetiology of sickle cell ischemic priapism: the pathophysiologic triad of hyperhaemolysis, hyperviscosity and hypercoagulability, and their therapeutic implications
Ahmed SG1, Ibrahim UA2
Abstract
Context: Majority of literature regarding pathophysiology of sickle cell ischaemic priapism (SCIP) is focused on penile veno-occlusion due to hyperhaemolysis-induced vasculopathy with little reference to SCD-associated hyperviscosity and hypercoagulability, each of which can also potentially cause penile veno-occlusion and SCIP.
Objective: This review has triple objectives to: (1)-reappraise role of hyperhaemolysis in aetiopathogenesis of SCIP; (2)-highlight roles of hyperviscosity and hypercoagulability in aetiopathogenesis of SCIP; and (3)-underscore aetiologically-determined therapeutic implications in managing SCIP due to hyperhaemolysis, hyperviscosity, and hypercoagulability.
Methodology: Online literature search was conducted using terms relevant to SCD and priapism. Only articles that reported aetiopathogenesis and/or management of SCD-associated priapism vis-à-vis hyperhaemolysis, hyperviscosity, and/or hypercoagulability were selected.
Results: SCIP has three aetiopathogenetic categories: (1)-hyperhaemolysis vis-à-vis penile venous vasculopathy; (2)-hyperviscosity vis-à-vis penile venous sludging; and (3)-hypercoagulability vis-à-vis corporal cavernosal thrombosis. Irrespective of aetiopathogenesis, short-term management of SCIP is prompt decompression to avert erectile dysfunction. However, long-term management differs with respect to aetiology. Aetiologically rational long-term management (ARLTM) for hyperhaemolysis-induced SCIP should be based on up-regulators of nitric oxide and PDE-5 (e.g., hydroxyurea or PDE-5 inhibitors). However, ARLTM for hyperviscosity-induced SCIP should aim at reducing microvascular endothelial-adhesion, sludging, and viscosity by using p-selectin inhibitors (crizanlizumab), while ARLTM for hypercoagulability-induced thrombotic SCIP should obviously be anticoagulation.
Conclusion: Hyperhaemolysis is the prototype and predominant aetiology of SCIP. However, veno-occlusive SCIP may occur due to any one of SCD-associated pathophysiologic triad of hyperhaemolysis, hyperviscosity, and hypercoagulability. While penile decompression remains universal short-term management of SCIP, long-term management should be rationally determined by aetiopathogenesis.
Key Words: Sickle Cell Disease, Priapism, Aetiopathogenesis, Hyperhaemolysis, Hyperviscosity, Hypercoagulability, Treatment
Introduction
The sickle haemoglobin (HbS) is a β-globin chain variant that evolved from the normal haemoglobin (HbA) as a result of a point mutation, which is caused by base transition from GAG (for glutamic acid) to GTG (for valine) at codon-6 of the β-globin gene on chromosome-11.1,2 In the heterozygous state, HbS gene is essentially asymptomatic and protects against severe malaria.3 However, sickle cell disease (SCD), which arises from homozygous inheritance of HbS gene or double heterozygosity of HbS gene with another haemoglobinopathy gene (e.g., HbSC, HbSD, HbSE, HbSO, and HbSβthal)1 is associated with significant morbidity and mortality.4,5 The pathophysiology of SCD is triggered by deoxy-HbS insolubility, polymerization, and red cell sickling.4,5 Sickled red cells cause vaso-occlusion, ischaemia, necrosis with reperfusion, and inflammation, resulting in painful vaso-occlusive crisis (VOC) and other forms of acute multi-organ damage.4,5 Moreover, sickled red cells undergo haemolysis, leading to nitric oxide deficient vasculopathy and chronic multi-organ damage.4,5 In addition, SCD is associated with infarctive autosplenectomy and impaired complement activation, resulting in immunosuppression and recurrent infections.4,5 The pathophysiology of SCD is thus shrouded in tissue necrosis, recurrent infections, haemolysis, and vasculopathy, all of which eventually lead to chronic anaemia, recurrent VOC, and other forms of acute and chronic multi-organ damage and dysfunctions.6,7 One of the most commonly affected organs in SCD is the penis, which is frequently afflicted and damaged by dysfunctional, non-erotic, unduly prolonged, and painful erection referred to as priapism.8,9
On the basis of the rate of arterial flow to the corpora, sickle cell priapism can be categorized as low or high flow priapism.8,9 Low flow priapism is caused by penile veno-occlusion, resulting in corporal compartment syndrome, ischaemia and pain, and is thus referred to as ‘sickle cell ischaemic priapism’ (SCIP).8,9 High flow priapism is usually caused by penile or perineal trauma leading to arterio-sinusoidal fistulas with increased corporal blood flow devoid of ischaemia or pain, and is thus referred to as ‘sickle cell non-ischaemic priapism’ (SCNIP).8,9 SCIP and SCNIP can be diagnosed and distinguished on the basis of clinical presentation, penile haemodynamic imaging, and gasometric and pH analyses of penile blood samples. Clinical distinction can be achieved by eliciting the presence of penile pain (in SCIP) or absence of penile pain (in SCNIP).8,9 In addition, haemodynamic imaging studies using color duplex ultrasonography would demonstrate decreased or absent cavernosal arterial flow in SCIP, whereas SCNIP is characterized by normal or high arterial flow velocities in cavernosal arteries.8,9 Moreover, gasometric and pH analysis of aspirated cavernous blood are important laboratory procedures for distinguishing between SCIP and SCNIP.8,9 In SCNIP, the aspirated blood is oxygenated and bright red in colour with blood gas and pH values showing partial pressure of oxygen (pO2)>90 mm Hg, partial pressure of carbon dioxide (pCO2)<40 mm Hg, and a pH of 7.40, all of which are consistent with normal arterial blood parameters at room air.8,9 Conversely, in SCIP the aspirated blood is hypoxic and dark red in colour with blood gas and pH values of pO2<40 mm Hg, pCO2>60 mm Hg, and pH<7.25.8,9
SCIP is the predominant (>95%) type of priapism in SCD, although isolated and rare cases of SCNIP had also been reported in only two patients with SCD.10 Paradoxically, the SCNIP in the aforementioned two cases of SCD arose apparently spontaneously without any antecedent trauma,10 which is at variance with the well known causative association between high flow priapism and penile or perineal trauma.8 Morphologically, SCIP is predominantly bicorporal (affecting the two corpora cavernosa only) in both children and adults, but in some cases tricorporal (affecting the two corpora cavernosa and corpus spongiosum) involvement had been reported especially in adults.11 Tricorporal SCIP tends to be more severe in presentation and worse in prognosis, and affects patients that often have pre-existing multi-organ damage and dysfunction.11
The most important demographic risk factor for SCIP is age. The onset of SCIP occurs in the pre-pubertal period and tends to be more recurrent and severe with increasing age and pubertal transition.12,13 The epidemiological trend shows that SCIP occurs less commonly with a prevalence of about 3.6% in children who are younger than 18 years of age.12,13 However, the prevalence of SCIP rises with increasing age, and it is reported to occur in up to 33% of SCD adolescents and adults,12,13 among whom SCIP tends to be more severe with higher risk of tricorporal involvement.11 This age-related epidemiological trend is consistent with the principal role of androgens in the physiology and pathophysiology of both normal and abnormal penile erections.14
Once SCIP becomes established, its clinical characteristics and consequences are determined by its duration, spontaneity of detumescence, recurrence or persistence. If an established SCIP is terminated by spontaneous detumescence in less than 4 hours after onset and is characteristically recurrent, the priapism is referred to as stuttering SCIP and it is less likely to cause ischaemic penile muscle injury.8,9 But if an established SCIP fails to resolve spontaneously and persists beyond 4 hours, the priapism is referred to as persistent SCIP and is more likely to cause penile muscle ischaemic injury.8,9 Consequently, SCIP is causally related to erectile dysfunction, which occurs with a prevalence rate that ranges from 11% to as high as 69.2% among various cohorts of SCD patients,12,13
Thus apart from its psychological effects, SCIP has a potentially profound impact on the reproductive performance of patients with SCD among whom SCIP it is the major cause of penile damage and erectile dysfunction.15 There is therefore the need to understand the aetiopathogenesis of SCIP. The overwhelming majority of the literature regarding the aetiopathogenesis of SCIP is focused on penile veno-occlusion due hyperhaemolysis-induced vasculopathy.16-22 However, a part from vasculopathy, the pathophysiology of SCD is strongly associated with hyperviscosity and hypercoagulability, both of which can independently cause penile veno-occlusion and potentially cause SCIP. But the literature is relatively silent on the aetiologic roles of hyperviscosity and hypercoagulability in the pathogenesis of SCIP. Hence, the aim of this overview is to present a comprehensive but concise narrative review with a triple objective to: (1) reappraise the role of hyperhaemolysis in the aetiopathogenesis of SCIP; (2) highlight the roles of hyperviscosity and hypercoagulability in the aetiopathogenesis of SCIP; and (3) underscore aetiologically determined therapeutic implications in the management of SCIP due to hyperhaemolysis, hyperviscosity, and hypercoagulability.
Methodology
Literature search and selection
Literature search was conducted using combinations of search terms relevant to SCD vis-à-vis aetiology, pathophysiology, treatment, and prevention of priapism, viz: ‘sickle cell disease, anaemia, priapism, aetiology, pathogenesis, pathophysiology, management, prevention, hyperhaemolysis, nitric oxide, vasculopathy, hyperviscosity, hypercoagulability, thrombosis, corpora cavernosa, corpus spongiosum’ in various combinations in PubMed, Medline, Bing, Google Scholar, and other online search engines. Literature search was unrestricted by year or place of publication. However, only articles that examined aetiology, pathogenesis, complications, management, and/or prevention of priapism in SCD were selected for this narrative review. Articles that concentrated on other aspects of SCD were excluded. A total of 73 relevant documents that were published between 1851 to 2023 were selected, which included 50 peer reviewed full articles, 21 peer reviewed case reports/case series, and 2 interim analyses of clinical trials as listed in the reference section.
Results
The literature revealed that the aetiology of SCIP falls into three main pathophysiologic categories, which include hyperhaemolysis vis-à-vis penile venous vasculopathy, hyperviscosity vis-à-vis penile venous sludging, and hypercoagulability vis-à-vis corpora cavernosal thrombosis. The aetiopathogenesis of SCIP due to hyperhaemolysis, hyperviscosity, and hypercoagulability, and their therapeutic implications in patients with SCD are outlined in Table 1 and expatiated in the discussion section.
Table 1: Aetiology, pathophysiology, and therapeutic implications of ischaemic priapism in sickle cell disease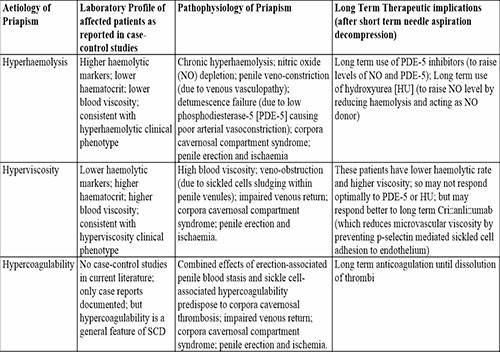
Discussion
The aetiopathogenesis of SCIP is based on penile veno-occlusion, corporal cavernosal over-distension, compartment syndrome, reduced arterial blood flow, and tissue ischaemia, which can be caused by veno-occlusive effects of any one of SCD-associated pathophysiologic triad of chronic hyperhaemolysis, chronic hyperviscosity, and chronic hypercoagulability. Nonetheless, the actual onset of SCIP may occur spontaneously or be triggered by a myriad of factors as described below.
a. Aetiopathogenesis of SCIP: Roles of physiological and non-physiological triggers of SCIP
SCIP can arise spontaneously or be triggered by a myriad of factors ranging from physiological to non-physiological factors. Commonly reported physiological triggers of SCIP include full bladder, sexual activity, as well as nocturnal or early morning erections during rapid eye movement sleep.8,12,23-29 Commonly reported non-physiological triggers of SCIP include sleep apnoea with nocturnal hypoxaemia and acidosis, acute asthmatic attack, acute infections, fever, dehydration, exposure to cold weather, use of recreational drugs such as cocaine and alcohol, or hormonal therapy with testosterone.8,12,23-29 In order to counteract some of these SCIP triggers, SCIP patients are encouraged to practice non-conventional relief measures at home before seeking hospital care. These measures include hydration (by drinking extra fluids), modest exercise, urination, application of warm or cold penile compresses, and masturbation with ejaculation.8,12,23-29 Coincidentally, most of the aforementioned non-physiological triggers of SCIP are also capable of triggering VOC.4 The observed similarity in the pattern of triggers between SCIP and VOC underscores the central role of sickling-induced acute microvascular occlusion (penile venules occlusion in the case of SCIP; and widespread tissue capillary occlusion in the case of VOC) as a common denominator in triggering the two clinically distinct vaso-occlusive disorders.
b. Aetiopathogenesis of SCIP: role of hyperhaemolysis-induced penile venous vasculopathy
Undoubtedly the overwhelming majority of studies on SCIP had pathophysiologically linked SCIP to impaired penile venous drainage due to hyperhaemolysis-induced vasculopathy.16-22 Hence, the aetiopathogenesis of SCIP is associated with the ‘hyperhaemolytic phenotype’ which is typically characterised by higher levels of haemolytic markers, greater depletion of nitric oxide, poorer endothelial and vascular dilatation functions, lower haematocrit, attenuated blood viscosity, and less frequent vaso-occlusive crisis (VOC).16-22 Nitric oxide is primarily produced by the neurones and endothelial cells via the enzymatic action of nitric oxide synthase upon arginine and oxygen as precursor molecules.30 SCIP is a major clinical manifestation of the hyperhaemolytic phenotype wherein freely released haemoglobin molecules quench nitric oxide.16-22 Nitric oxide is an essential molecule in the maintenance of endothelial function, vascular muscle tone, and vascular patency, all of which are important in the initiation, regulation, and termination of normal penile erection.30-32 Moreover, nitric oxide activates guanylate cyclase thus stimulating cyclic guanosine monophosphate (cGMP) production, which results in the activation of protein kinase-G.30-32 Activation of protein kinase-G causes penile arterial dilation, smooth muscle relaxation, compression of penile emissary veins, corpora cavernosal blood pooling, and penile erection.30-32 In addition, nitric oxide-induced cGMP up-regulates the expression of phosphodiesterase type-5 (PDE-5), which is a key enzyme associated with detumescence through degradation of cGMP.30-32 The process of detumescence starts soon after ejaculation or cessation of sexual stimulation, and is mediated by vasoconstriction of penile arteries (which reduces arterial inflow) and subsequent decompression of emissary veins (which increases penile venous drainage), both of which cause the penis to return to its base line flaccid state.31,32 The process of detumescence is significantly impaired at low level of PDE-5. Low level of PDE-5 occurs as a consequence of haemolysis-induced depletion of nitric oxide, which is physiologically required for up-regulation of cGMP and expression of PDE-5.31,32
Therefore, chronic nitric oxide depletion has dual adverse effects on penile function by causing vasculopathy16-22 and by reducing PDE-5 levels.31-33 On the one hand, penile veno-occlusive vasculopathy causes veno-endothelial dysfunction, veno-constriction, and impaired corporal venous drainage, all of which eventually lead up to full blown priapism.16-22 On the other hand, low PDE-5 level alters the set point for PDE-5 homeostasis, leading to detumescence failure due to inability to terminate normal penile erection via vasoconstriction of penile arteries.31-33 Thus chronic hyperhaemolysis-induced depletion of nitric oxide in SCD can cause priapism via the separate or combined effects of ‘veno-occlusive vasculopathy’16-22 or ‘low PDE-5-induced impairment of detumescence’.31-33 Interestingly, the relationship between low PDE-5 and poor detumescence31-33 forms the basis of therapeutically ‘rational’ but apparently ‘paradoxical’ use of PDE-5 inhibitors (well known medications for erectile dysfunction) in the prophylactic management of hyperhaemolysis-induced recurrent SCIP in patients with SCD as described in the next paragraph.
c. Therapeutic implications of hyperhaemolysis-induced SCIP
In the short term, all acute cases of SCIP (irrespective of etiology) should be promptly decompressed by needle aspiration with or without saline or alpha-adrenergic agonist irrigation in order to avoid time-dependent risk of corporal ischemic injury and erectile dysfunction.23 Aetiologically rational long term management of recurrence of hyperhaemolysis-induced SCIP is currently based on appropriate use of medications that specifically target and ameliorate the pathophysiologic roles of hyperhaemolysis, nitric oxide quenching, vasculopathy, and low level of PDE-5. For example, long term treatment of hyperhaemolysis-induced SCIP with PDE-5 inhibitors leads to steady rise in cGMP, which boosts cGMP-promoter activity, induces tissue expressions of PDE-5, and eventually normalize and sustain penile detumescence mechanisms.31-33 The aforementioned cascade of biochemical events explains the ‘paradoxical’ effectiveness of chronic administration of carefully ‘regimented schedule’ of low doses of short acting PDE-5 inhibitors.34,35 In a longitudinal study, patients with recurrent SCIP were enrolled in a regimented therapy with low doses of short-acting PDE5 inhibitors using an initial oral daily morning doses of 25mg of sildenafil, which was unassociated with sexual stimulation or activity, with the possible option of dose escalation depending on individual patient response.35 The regimented therapy had dual precautionary reasons behind the choice of short acting PDE-5 and the morning period as the time of administration. First, the reason behind the use of low doses was to mitigate potential dose-dependent risk of PDE-5 inhibitor induced priapism or VOC.35-37 Second, the reason behind morning time administration of short acting PDE5 inhibitors was based on the rapid pharmacokinetic elimination of the drug in order to avert aggravating the risk of nocturnal priapism possibly arising from sleep-related erections or sexual intercourse.35,38 Moreover, long term use of hydroxyurea, a drug that improves vascular endothelial function by diminishing haemolytic rate, reducing nitric oxide quenching (nitric oxide sparing effect), and acting as nitric oxide donor, has been shown to reduce the frequency of recurrent episodes in patients with hyperhaemolysis-induced SCIP.39 Research is ongoing regarding the possibility of using haptoglobins to scavenge hyperhaemolysis-derived free haemoglobin and prevent it from binding and quenching nitric oxide; a therapeutic strategy that will go a long way in preventing hyperhaemolysis-induced vasculopathy and reducing the frequency of recurrent SCIP.40
d. Aetiopathogenesis of SCIP: the role of hyperviscosity-induced penile venous sludging
Despite the popularity of the role of hyperhaemolytic vasculopathy in the aetiopathogenesis of SCIP,16-22 a few cohort studies41-43 had revealed that certain cases of SCIP might not be causally related to hyperhaemolysis for two reasons. First, one cohort study had shown that in comparison to patients without SCIP, patients with SCIP were found to have similar rates of VOC, levels of haemolytic markers, and haematocrit.41 This study did not find significant pathophysiologic correlation between hyperhaemolysis and SCIP, and suggested that non-hyperhaemolytic factors may be involved in the causation of priapism in some patients with SCIP.41 Second, two other cohort studies had shown that patients with SCIP had even higher levels of haematocrit and haemoglobin concentrations vis-à-vis their counterparts without SCIP.42,43 The findings of these two studies underscored the pathophysiologic significance of hyperviscosity in the causation of SCIP, and suggested that some cases of SCIP were associated with the ‘hyperviscosity phenotype’ and were caused by hyperviscosity-induced sludging and penile veno-obstruction rather than vasculopathy-induced penile veno-constriction.42,43 It is an established fact that SCD pathophysiology is associated with sickling-induced hyperviscosity.44 Hence, we believe that the dual hyperviscosity effects of ‘sickled red cells’ and ‘relatively higher levels of haematocrit’ were responsible for sludging and penile veno-obstruction leading up to the development of SCIP in SCD patients with haematological features of ‘hyperviscosity phenotype’ as reported in the two aforementioned studies.42,43 It is therefore very plausible that hyperviscosity is an important aetiologic factor for SCIP among patients with hyperviscosity phenotype. Indeed, the role of hyperviscosity as an independent aetiologic factor for sludging and veno-obstructive ischaemic priapism had been documented in non-SCD patients with polycythemia,45 leucocytosis,46,47 thrombocytosis,48 or hyperproteinemia.49
e. Therapeutic implications of hyperviscosity-induced SCIP
As earlier mentioned, in the short term, all acute cases of SCIP (irrespective of etiology) should be promptly decompressed by needle aspiration with or without saline or alpha-adrenergic agonist irrigation in order to avoid time-dependent risk of corporal ischemic injury and erectile dysfunction.23 However, we believe that hyperviscosity-induced SCIP may have two therapeutic implications with respect to the use of PDE-5, hydroxyurea, and/or transfusion therapy as elucidated in subsequent paragraphs.
The first therapeutic implication is based on the fact that hyperhaemolysis and nitric oxide quenching are not prominent pathophysiologic features of hyperviscosity-associated SCIP. Hence, patients affected by hyperviscosity-associated SCIP may not respond optimally to nitric oxide up-regulating effects of PDE-5 inhibitors,34,35 anti-haemolytic (nitric oxide sparing) effect of hydroxyurea,39 and nitric oxide donor effect of hydroxyurea39 as previously reported in patients with hyperhaemolysis-induced SCIP.34,35,39 We therefore believe that aetiologically rational long term management of recurrence of hyperviscosity-induced SCIP should be based on medications that would specifically ameliorate the background hyperviscosity-related pathophysiology. Suffices to note that in addition to haematocrit, SCD hyperviscosity is significantly contributed by microvascular sludging due to intercellular adhesion between platelets, leucocytes, sickled red cells, and the microvascular endothelium.50 We thus reckon that patients with hyperviscosity-induced SCIP would probably show better response to drugs that can reduce microvascular viscosity and decrease sludging within penile venous circulation. To the best of our knowledge, crizanlizumab is probably the only ‘SCD drug in current clinical use’ that can ‘directly’ improve microvascular viscosity, rheology, and blood flow by inhibiting p-selectin mediated intercellular adhesion between platelets, leucocytes, sickled red cells, and the microvascular endothelium as reported in the interim analysis of the SMAART CRIZ clinical trial.51 We thus believe that crizanlizumab is an aetiologically rational drug for managing hyperviscosity-induced SCIP. Interestingly, previous studies had already demonstrated the safety and efficacy of crizanlizumab in reducing the frequency of recurrent SCIP as reported in one case series52 and the interim analysis of the SPARTAN clinical trial.53
The second therapeutic implication is related to the fact that in comparison to patients with hyperhaemolysis-associated SCIP, patients with hyperviscosity-associated SCIP tend to have relatively higher levels of haematocrit. Hence, patients with hyperviscosity-associated SCIP would be at higher risk of transfusion-induced hyperviscosity-related neurological complications such as the ASPEN syndrome.54,55 Previously reported cases of ASPEN syndrome occurred as a result of inadvertent induction of iatrogenic hyperviscosity, which can be effectively prevented by keeping post-transfusion haematocrit at less than 30%.56 This calls for closer observation (to ensure that post-transfusion haematocrit does not exceed 30%) if and when exchange transfusion is warranted in the clinical care of patients with hyperviscosity-associated SCIP.56 This precautionary measure to avoid transfusing patients with SCD to haematocrit levels beyond 30% (due to its hyperviscosity consequences) should be observed, not only in priapism, but in all cases of SCD morbidities requiring transfusion therapy.56 There is no doubt that the currently available literature suggests that hyperhaemolysis-induced SCIP is the prototype and predominant form of priapism in SCD.16-22 Nonetheless, it is important for SCD care givers to as far as possible identify (via critical review of clinical and laboratory profiles of SCIP patients) the relatively few cases of hyperviscosity-induced SCIP42,43 in order to preempt the aforementioned potential therapeutic implications.
f. Aetiopathogenesis of SCIP: role of hypercoagulability-induced corporal thrombosis
Cases of priapism due to partial or complete thrombosis of the corpora cavernosa had been reported in the literature. Partial priapism is a rare urological condition due to partial segmental thrombosis of the proximal part of corpus cavernosum (PSTPCC). PSTPCC in non-SCD patients is usually seen in haemostatically normal young adults among whom it could occur idiopathically or be triggered by trauma, cocaine, marijuana, alcohol, dehydration, surgery, malignancy, or long distance flights, but it had also been strongly associated with hereditary thrombophilias such as hyper-homocyteinemia and factor-V Leiden associated protein-C resistance.57-59 The most common symptoms of PSTPCC described in the literature are perineal and proximal penile pain together with a palpable tender swelling of the thrombosed and rigid proximal part of one or both corpora cavernosa, which is usually unassociated with obvious penile erection.60 However, depending on the position of PSTPCC and its effect on the venous drainage of the distal part of the corpora cavernosa, the penis may appear semi-erect.60 The literature is silent on the occurrence of complete thrombotic priapism due to complete thrombosis of the corpora cavernosa in non-SCD persons even among those with hereditary thrombophilias. Nonetheless, both partial and complete thrombotic priapism have been reported in patients with SCD, the pathophysiology of which is strongly correlated with hypercoagulability as elucidated in subsequent paragraphs.
Hypercoagulability in patients with SCD is due to combined effects of necrosis-induced increased expression of tissue factor, sickling-induced exposure of red cell membrane-derived negatively charged phosphatidylserine, inflammation-induced activation of clotting factors, defective fibrinolysis, and decreased levels of natural anticoagulants.61-64 Moreover, SCD is also associated with chronic inflammation-induced vascular endothelial damage.65 Consequently, patients with SCD are prone to develop thrombotic complications within the vasculature of any part of their body.61-64 But, in particular, the corpora cavernosa of patients with SCD should be at higher risk of thrombosis than any part of the SCD patient body. This is because of the triple concert between SCD-induced hypercoagulability,61-64 SCD-induced endothelial injury,65 and erection-induced blood stasis in the corpora,66 all of which undesirably constitute the ‘complete set’ of Virchow’s thrombotic triad67 within the erect penis of the SCD patient. It is thus presumable that even physiologically normal erections (due to sexual intercourse, nocturnal/morning tumescence, or full bladder) in SCD patients may trigger corporal cavernosal thrombosis, obstruct venous outflow, and ‘metamorphous’ into a full blown SCIP. In view of the aforementioned reasons, we expected corpora cavernosal thrombosis to be a frequent aetiologic factor in the causation of SCIP. Paradoxically, despite the high risk of thrombotic morbidities in SCD in general,63 coupled with the potentially prothrombotic effect of blood stasis within the erect penis66 of SCD patients, the role of corpora cavernosal thrombosis in the aetiology of SCIP is surprisingly scanty in the literature. We believe this apparent scantiness of literature suggests under-reporting and/or under-diagnosis, since not all SCD care centres in the developing world (which bears the heaviest burden of SCD)68 may have the necessary scanning and imaging facilities for detecting penile thrombi69 in SCD patients with SCIP. Nevertheless, at least two cases of partial priapism due to PSTPCC had been described in patients with SCD.70,71 Partial priapism due to PSTPCC in SCD carries relatively low risk of ischemic injury and erectile dysfunction, but the risk would escalate if it progresses to complete corpora cavernosal thrombosis, which can lead to full blown SCIP as previously reported in three patients with SCD,71-73 one of which had severe corpora cavernosal necrosis that necessitated corporectomy.73
g. Therapeutic implications of hypercoagulability-induced thrombotic SCIP
The occurrence of both partial and complete thrombotic priapism in SCD underscores the important role of hypercoagulability in the pathogenesis of priapism in patients with SCD. But the actual incidence of thrombotic priapism in SCD can only be known when penile scanning becomes a routine procedure for all patients presenting with SCIP in low resource countries, which carry the heaviest clinical burden of SCD.68 Ideally, every SCD patient with SCIP should be promptly evaluated by penile scanning and imaging procedures,69 in order to detect any thrombi and proffer appropriate management.70-73 Prompt decompression by needle aspiration with or without saline or alpha-adrenergic agonist irrigation remains the most important universal short term management against the risk of corporal ischemic injury and erectile dysfunction in all cases of SCIP.23 However, the aetiologically rational long term management for hypercoagulability-induced partial or complete thrombotic priapism in patients with SCD is obviously anticoagulation until complete dissolution of corpora cavernosal thrombi is radiologically confirmed.70-73 Moreover, even after complete dissolution of corpora cavernosal thrombi, affected patients should be carefully assessed for possible risks of recurrence, which may require an extended period of prophylactic anticoagulation for a duration to be clinically determined by the attending health care personnel.
Conclusion
Hyperhaemolysis is the prototype and predominant aetiology of SCIP. Nevertheless, SCIP may occur due to any one of SCD-associated aetiopathogenetic triad of hyperhaemolysis, hyperviscosity, and hypercoagulability. Irrespective of the aetiopathogenesis, the short term management of SCIP is universally based on prompt corpora cavernosal aspiration and decompression in order to avert tissue necrosis, fibrosis and erectile dysfunction. However, the long term management of recurrence of SCIP differs with respect to aetiopathogenesis. On the one hand, aetiologically rational long term management for hyperhaemolysis-induced SCIP is to minimize haemolysis, raise the level of nitric oxide, mitigate vasculopathy, and increase the activity of PDE-5 through the use of hydroxyurea and/or PDE-5 inhibitors. On the other hand, aetiologically rational long term management for hyperviscosity-induced SCIP should aim at reducing microvascular viscosity by minimizing intercellular and sickled cell-endothelial adhesions through the use of p-selection inhibitors such as crizanlizumab. And obviously, the aetiologically rational long term management for hypercoagulability-induced thrombotic SCIP should be adequate anticoagulation until complete dissolution of the corpora cavernosal thrombi is radiologically confirmed. We recommend that every case of SCIP should be subjected to thorough clinical, haematological, and radiological evaluations in order to assess the separate or combined aetiopathogenetic roles of hyperhaemolysis, hyperviscosity, and hypercoagulability. This approach will help in determining the most appropriate and aetiology-specific therapeutic measures for each patient with SCIP.
Disclosure: Authors declare no conflict of interest.
Funding: None
References
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1993; 6: 215-262.
- Kaul DK, Fabry ME, Nagel RL. The pathophysiology of vascular obstruction in the sickle syndromes. Blood Rev 1996; 10: 29-44.
- Fleming AF, Storey J, Molineaux L, Iroko EA, Attai ED. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol 1979; 73: 161-172.
- Ahmed SG, Ibrahim UA. A compendium of pathophysiologic basis of etiologic risk factors for painful vaso-occlusive crisis in sickle cell disease. Niger J Basic Clin Sci 2017; 14: 57-77.
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994; 330: 1639-1644.
- Vichinsky E. Chronic organ failure in adult sickle cell disease. The American Society of Hematology Education Programme Book 2017; 1: 435-439.
- Hassell KL, Eckman JR, Lane PA. Acute multi-organ failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 1994; 96: 155-162.
- Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: Pathogenesis, epidemiology and management. J Sex Med 2010; 7: 476-500.
- AlDallal S, AlDallal N, Alam A. Sickle cell-induced ischemic priapism. Cogent Medicine 2016; 3: 1268357. DOI:10.1080/2331205X.2016.1268357.
- Ramos CE, Park JS, Ritchey ML, Benson GS. High flow priapism associated with sickle cell disease. J Urol 1995; 153: 1619-1621.
- Sharpsteen JR, Powars D, Johnson C, Rogers ZR, Williams WD, Posch RJ. Multisystem damage associated with tricorporal priapism in sickle cell disease. Am J Med 1993; 94: 289-295.
- Alvaia MA, Maia HA, Nelli AM, Guimarães CO, Carvalho ES, Netto JM, et al. Prevalence of priapism in individuals with sickle cell disease and implications on male sexual function. Einstein 2020; 18: eAO5070.
- Idris IM, Abba A, Galadanci JA, Mashi SA, Hussaini N, Gumel SA, et al. Men with sickle cell disease experience greater sexual dysfunction when compared with men without sickle cell disease. Blood Adv 2020; 4: 3277-3283. DOI:10.1182/bloodadvances.2020002062.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am 2005; 32: 379-v.
- Smith-Whitley K. Reproductive issues in sickle cell disease. Blood 2014; 124: 3538-3543.
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical sub-phenotypes. Blood Rev 2007; 21: 37-47.
- Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood 2005; 106: 3264-3267.
- Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, Morris CR, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 2006; 107: 2279-2285.
- Ozahata MC, Page GP, Guo Y, Ferreira JE, Dinardo CL, Carneiro-Proietti ABF, et al. Clinical and genetic predictors of priapism in sickle cell disease: Results from the recipient epidemiology and donor evaluation study III Brazil cohort study. J Sex Med 2019; 16: 1988-1999.
- Yau NA, Mustafa FG, Abdulqadir I. Sickle cell anaemia related priapism in Kano, North- Western, Nigeria: Re-emphasizing the important role of haemolysis. J Med Res 2023; 9: 84-87.
- Taylor JG, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT. Chronic hyperhemolysis in sickle cell anemia: Association of vascular complications and mortality with less frequent vaso-occlusive pain. PLoS ONE 2008; 3: e2095. DOI:10.1371/journal.pone.
- Taylor JG, Nolan VG, Kato GJ, Gladwin M, Steinberg MH. The hyperhemolysis phenotype in sickle cell anemia: Increased risk of leg ulcers, priapism, pulmonary hypertension and death with decreased risk of vaso-occlusive events. Blood 2006; 108: 787.
- Olujohungbe A, Burnett AL. How I manage priapism due to sickle cell disease. Br J Haematol 2013; 160: 754-765. DOI:10.1111/bjh.12199.
- Dupervil B, Grosse S, Burnett A, Parker C. Emergency department visits and inpatient admissions associated with priapism among males with sickle cell disease in the United States, 2006-2010. PLoS ONE 2016; 11: e0153257.
- Adeyoju AB, Olujohungbe AB, Morris J, Yardumian A, Bareford D, Akenova A, et al. Priapism in sickle-cell disease; incidence, risk factors and complications-an international multicentre study. BJU Int 2002; 90: 898-902.
- Conrad ME, Perrine GM, Barton JC, Durant JR. Provoked priapism in sickle cell anemia. Am J Hematol 1980; 9: 121-122.
- Jiva T, Anwer S. Priapism associated with chronic cocaine abuse. Arch Intern Med 1994; 154: 1770.
- Salloum E, Ohri A, Bartlett F, Ivey T, Savona S. Priapism in sickle cell disease: Possible contributory effect of cocaine use. Arch Intern Med 1993; 153: 2287.
- Slayton W, Kedar A, Schatz D. Testosterone induced priapism in two adolescents with sickle cell disease. J Pediatr Endocrinol Metabol 1995; 8: 199-203. DOI:10.1515/JPEM.1995.8.3.199.
- Lundberg JO, Weitzberg E. Nitric oxide signaling in health and disease. Cell 2022; 185: 2853-2878.
- Hudnall M, Reed-Maldonado AB, Lue TF. Advances in the understanding of priapism. Transl Androl Urol 2017; 6: 199-206.
- Musicki B, Burnett AL. Mechanisms underlying priapism in sickle cell disease: targeting and key innovations on the preclinical landscape. Expert Opin Ther Targets 2020; 24: 439-450.
- Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. PNAS 2005; 102: 1661-1666.
- Nardozza-Junior A, Cabrini MR. Daily use of phosphodiesterase type 5 inhibitors as prevention for recurrent priapism. Rev Assoc Med Bras 2017; 63: 689-692.
- Hou LT, Burnett AL. Regimented phosphodiesterase type-5 inhibitor use reduces emergency department visits for recurrent ischemic priapism. J Urol 2020; 205: 545-553.
- Rezaee ME, Gross MS. Are we overstating the risk of priapism with oral phosphodiesterase type 5 inhibitors? J Sex Med 2020; 17: 1579-1582.
- Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 2011; 118: 855-864.
- Figueiredo MS, Cançado RD, Pollack-Filho F, Arruda MMAS, Sato PVJR, Tufik S, et al. Priapism is associated with sleep hypoxemia in sickle cell disease. J Urol 2012; 188: 1245-1251.
- Morrison BF, Hamilton P, Reid M. Effect of hydroxyurea on priapism in men with sickle cell disease. West Indian Med J 2017; 66: 518-520.
- Pereira PS, Pereira DA, Calmasini FB, Reis LO, Brinkman N, Burnett AL, et al. Haptoglobin treatment contributes to regulating nitric oxide signal and reduces oxidative stress in the penis: A preventive treatment for priapism in sickle cell disease. Front Physiol 2022; 13: 961534.
- Madu AJ, Ubesie A, Ocheni S, Chinawa J, Madu KA, Ibegbulam OG, et al. Priapism in homozygous sickle cell patients: important clinical and laboratory associations. Med Princ Pract 2014; 23: 259-263.
- Ahmed SG, Ibrahim UA, Hassan AW. Haematological parameters in sickle cell anaemia patients with and without priapism. Ann Saudi Med 2006; 26: 439-443.
- Adedeji MO, Onuora VC, Ukoli FA. Haematological parameters associated with priapism in Nigerian patients with homozygous sickle cell disease. Am J Trop Med Hyg 1988; 91: 157-159.
- Perazzo A, Peng Z, Young YN, Feng Z, Wood DK, Higgins JM, et al. The effect of rigid cells on blood viscosity: Linking rheology and sickle cell anemia. Soft Matter 2022; 18: 554-565.
- Munarriz R, Kim NN, Traish A, Goldstein I. Priapism. In: Wessells H, Mc Aninch JW. (eds). Urological emergencies. Current Clinical Urology. Humaris Press. 2005; 213-224.
- Gupta G, Kumar D, Trivedi M. Acute lymphoblastic leukemia in a child presenting primarily with priapism. J Indian Assoc Pediatr Surg 2020; 25: 52-54.
- Gaye O, Thiam NM, Cassell A, Gueye SM, Sow Y, Fall B, et al. Unusual presentation of priapism associated with acute and chronic myeloid leukemia in two patients: Emergency management. Case Rep Urol 2020; Article ID 4982432: 5 pages.
- Verma SP, Kumar N, Jain M, Tripathi AK. Essential thrombocythemia presenting with recurrent priapism: A case report and review of literature. Am J Case Rep 2020; 21: e924455.
- Sahu KK, Mishra K, Dhibar DP, Ram T, Kumar G, Jain S, et al. Priapism as the presenting manifestation of multiple myeloma. Indian J Hematol Blood Transfus 2017; 33: 133-136.
- Klug PP, Lessin LS, Radice P. Rheological aspects of sickle cell disease. Arch Intern Med 1974; 133: 577-590.
- Guzzardo GG, Chitlur M, Hines PC, Zaidi AU, Borhan R, Cinciarelli K, et al. Smaart Criz: Sickle cell mechanisms of activation, adhesion, rheology, and thrombosis (SMAART) in response to P-selectin inhibition. Blood 2023; 142: 2528.
- Idowu M, Garcia RL, Sule OB. Successful treatment of SCD-related priapism with Crizanlizumab: A case series. J Invest Med High Impact Case Reports 2023; 11: 1-4.
- Anderson AA, El Rassi F, Debaun MR, Idowu M, Kanter J, Adam S, et al. Interim analysis of a Phase-2 trial to assess the efficacy and safety of Crizanlizumab in sickle cell disease patients with priapism (spartan). HemaSphere 2023; 7: e54376db.
- Rackoff WR, Ohene-Frempong K, Month S, Scott JP, Neahring B, Cohen AR. Neurologic events after partial exchange transfusion for priapism in sickle cell disease. J Pediatr 1992; 120: 882-885.
- Siegel JF, Rich MA, Brock WA. Association of sickle cell disease, priapism, exchange transfusion and neurological events: ASPEN syndrome. J Urol 1993; 150: 1480-1482.
- Ballas SK, Lyon D. Safety and efficacy of blood exchange transfusion for priapism complicating sickle cell disease. J Clin Apher 2016; 31: 5-10.
- Blaut S, Schneider M, Zschuppe E, Günl U, Steinbach F. Partial unilateral penile thrombosis of corpus cavernosum due to hyperhomocysteinemia: Case report and references. Der Urologe 2008; 47: 748-752.
- Gomez Gomez E, Campos Hernandez JP, Cazalilla M, Trivino F, Barbudo J, Prieto R, et al. Partial thrombosis of the corpus cavernosum: should we dig deeper into coagulopathy disorders? Andrologia 2016; DOI:10.1111/and.12572.
- Dubois F, Lesur G, Azzouzi AR, Beurrier P, Chautard D. Partial Thrombosis of the Corpus Cavernosum. Must a clotting disorder be systematically investigated? Progrès en Urologie 2007; 17: 866-868.
- Christodoulidou M, Parnham A, Ramachandran N, Muneer A. Partial segmental thrombosis of the corpus cavernosum with perineal pain. BMJ Case Rep 2016; 2016: bcr2016217748.
- Faesa Alhawiti NM. Hemostatic alteration in sickle cell disease: Pathophysiology of the hypercoagulable state. King Khalid Univ J Health Sci 2021; 6: 1-5.
- Sparkenbaughb EM, Pawlinskic R. Hypercoagulable state in sickle cell disease. Clin Hemorheol Microcirc 2018; 68: 301-318.
- Brunson A, Keegan T, Mahajan A, White R, Wun T. High incidence of venous thromboembolism recurrence in patients with sickle cell disease. Am J Hematol 2019; 94: 862-870.
- Phillips G, Mitchell LB, Pizzo SV . Defective release of tissue plasminogen activator in patients with sickle cell anemia. Am J Hematol 1988; 29: 52-53.
- Hoppe CC. Inflammatory mediators of endothelial injury in sickle cell disease. Haematol Oncol Clin 2014; 28: 265-286.
- Kelly DA. Penises as variable volume hydrostatic skeletons. Ann NY Acad Sci 2007; 1101: 453-463. DOI:10.1196/annals.1389.014.
- Virchow R. Über die Erweiterung kleinerer Gefäβe. Arch Pathol Anat Physiol Klin Med 1851; 3: 427-462.
- Global Burden of Diseases Collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000-2021: a systematic analysis from the global burden of disease study 2021. Lancet Haematol 2023; 10: e585-99.
- Asbach P, Oelrich B, Haase O, Lenk SV, Loening SA. Acute partial segmental thrombosis of the corpus cavernosum: imaging findings on ultrasound, computed tomography, and magnetic resonance imaging. Clin Imaging 2008; 32: 400-402.
- Malallahn M, Al-Rashed H, Al-Terki A, Al-Shaiji T. Partial priapism: A rare presentation of sickle cell anemia. New Horizons in Clin Case Rep 2017; 1: 9-10.
- İlhan G. Thrombosis of corpus cavernosum in a sickle cell anemia patient with priapism Cukurova Med J 2017; 42: 357-359. DOI:10.17826/cutf.322958.
- Sawan E. Priapism due to thrombosis in sickle cell anemia. Clin Proc Child Hosp Dist Columbia. 1947; 3: 241-243.
- Yang YM, Donnell CA, Farrer JH, Mankad VN. Corporectomy for intractable sickle-associated priapism. Am J Med Sci. 1990; 300: 231-233.