Management of diaphragmatic eventration in a low-income setting: A case report
Nwafor IA, Agagwuncha N, Ufio B. Eze JC
Abstract
Diaphragmatic eventration, is a rare pathology that is characterized by abnormal elevation of the diaphragm and abdominal contents residing within thoracic cavity, though separated from the lungs by the thin or membraneous diaphragm. It may be asymptomatic but may result in dyspnoea, heaviness in the chest and hearing of bowel sounds in the chest. We report a 63 year old man who presented to our service, 10 years history prior, of heaviness in the left hemithorax and whose chest x-ray revealed an abnormally elevated dome of the left hemidiaphragm in the chest (eventration). He was worked up for left thoracotomy, plication of the diaphragm with uneventful recovery postoperatively and disappearance of symptoms.
Keywords: Eventration, dyspnoea, plication, thoracotomy, diaphragm
Introduction
Diaphragmatic eventration is a rare pathology, which may be congenital or acquired. It occurs as a result of defect in the muscular portion of the diaphragm, which appears attenuated and membranous, maintaining its normal attachments and its anatomical continuity. It has been attributed to abnormal myoblast migration to the septum transversum and the pleuroperitoneal membrane.1 It can be unilateral, bilateral, partial or total, symptomatic or asymptomatic. It is commoner in the left hemidiaphragm than the right and commoner in males than females and affects all age groups, adult and children alike.2
The ‘weak’ diaphragm leads to a decrease of its movements and even paradoxical motion may occur, causing a poor lung expansion and decreased blood flow, generating a hypoxi-mediated vasoconstriction. This elevation of the diaphragmatic dome to the chest also allows the protrusion of abdominal contents to the thoracic cavity, which characterizes the symptomatology.3
Clinically, the presentation has variable spectrum, depending on the type of eventration (whether total or partial) and the greater or lesser elevation and function of the diaphragmatic dome. In the adult unlike in children the presentation is largely asymptomatic, except if it is total where patient may complain of digestive symptoms, such as abdominal pain, vomiting and flatulence.4 In children, orthopnoea, recurrent respiratory infections, bad weight progression and intolerance to stress can occur while in the newborn, acute respiratory distress syndrome may occur. Diagnosis can be confirmed with postero-anterior chest radiograph and or abdominal ultrasound.4,5
Therapeutically, the aims are to increase the thoracic volume and vital capacity and reduce the paradoxical movement of the diaphragm, which subsequently, solves the problem by minimizing the clinical manifestations and making more efficient ventilation.6-8 Options of treatment depend on the clinical manifestation. The management of this pathology depends on the degree of respiratory distress. In asymptomatic cases and with mild respiratory distress, supplemental oxygen is sufficient, while in cases of serious and persistent respiratory distress or where mechanical ventilation is needed, surgical fixation of the diaphragm (plication), which is surgical procedure of choice is indicated.9-11
Case summary
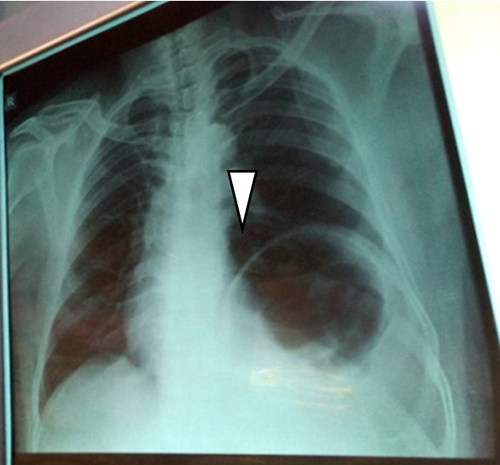
Figure 1: Preoperative postero-anterior chest radiograph showing the elevated dome of left hemidiaphragm (white arrow)
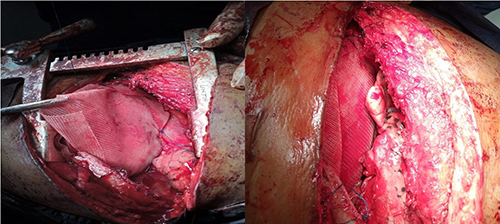
Figure 2 (a & b): Intraoperative procedure (application of prolene mesh)
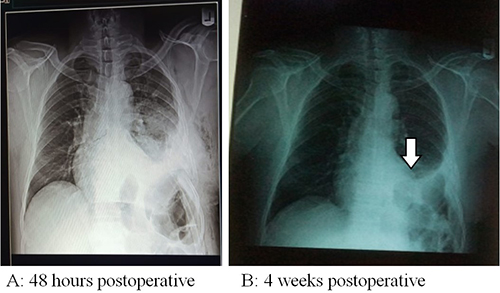
Figure 3: Postoperative chest x-ray with white arrow pointing to the dome of left hemidiaphragm
A 59 year old businessman presented to our service with four years history of left chest pain which was tight in nature, moderately severe and aggravated with ingestion of food, with no relieving factors. Pain did not radiate to any other parts of the body. There was no associated gastrointestinal, urological or musculo-skeletal symptoms. He was not hypertensive or diabetic. Clinical examination revealed a middle aged man in no obvious distress. He had normal vital signs. However, there was hyper-resonant percussion note on the left hemithorax with reduced breath sound on that side. No significant findings on the abdomen.
His haematological, serological (retroviral screening, hepatitis B surface antigen) and biochemical parameters were normal. Chest x-ray showed a highly elevated left hemi-diaphragmatic dome. Diagnosis of eventration of diaphragm was made and he was counseled and told that the treatment would be surgical and he gave a written consent for preoperative work-up and left poster-lateral thoracotomy. Intraoperatively, the left hemi-diaphragm was found to be very thin (<0.5cm thick) without muscle; and displaced superiorly in the chest with the fundus reaching the third intercostal space and intraperitoneal organs residing in the 'chest’ region. Subsequently, plication of the left hemi-diaphragm was done using polypropylene (prolene) mesh. This resulted in pushing the intraperitoneal organs down to the ‘abdominal’ region. See figure 3A. Postoperative course was uneventful. Postoperative chest x-ray revealed marked downward displacement of the elevated dome of the left hemidiaphragm. Since then, patient has been symptom free.
Discussion
The diaphragmatic eventration is responsible for significant morbidity in children and adults. In cases of severe disease, surgical intervention may be required.12 Various approaches have been employed for diaphragmatic plication, including open transthoracic approaches (various thoracotomies, sternotomy, hemi-clam shell incision), thoracoscopic approaches (various numbers, lengths, and location of ports), mimimally invasive approaches (small thoracotomies and thoracoscopically assisted mini thoracotomies), and open transabdominal, or laparoscopic approaches.13 In the index case, open thoracotomy was used. Patient was initially placed in supine position, given general anaesthesia with cuffed endotracheal intubation. He was turned into right lateral decubitus position, cleansed and draped for left posteriolateral thoracotomy. Intraoperatively, the findings were highly elevated dome of the left hemidiaphragm that was very thin (<0.5 cm thick).
All techniques aim to reduce the abundant diaphragmatic surface and lower the diaphragmatic dome.13 Various suturing methods have been used, including (buttressed or not) interrupted horizontal mattress sutures, multiple parallel U sutures, figure of eight sutures, continuous running sutures, and endostaplers(minimal access method).4
In our index case, the diaphragm was split open centrally, and intraperitonal structures, inspected. Thereafter, the two flaps were double breasted (overlapped), anterior flap over the posterior. The flaps were tightly reinforced with nonabsorbable suture (nylon 2). Subsequently, Prolene mesh was used to reinforce the flaps using nonabsorbable sutures. See figures 2 a and b. Ultimately, thoracotomy wound was closed in layers with chest drain in situ. On the 5th postoperative day, the chest drain was removed following 2nd postoperative day chest x-ray. See figure 3.
In the other literary works, different methods have been adopted. According to early, and a few recent reports, excision of the abundant diaphragm was performed. In 1959, Christensen5 reported oval radial excision and suturing in 2 or 3 layers with nonabsorbable material, through an open transthoracic approach. In 1970, Thomas12 reported opening of the membranous portion of the diaphragm, freeing of the adhesions to the underlying abdominal viscera, resection of the excess membranous portion, and a two-layer overlapping approximation with nonabsorbable interrupted sutures, through an open transthoracic approach. In 2006, Di Giorgio et al,14 reported dome incision and excision of a portion of the eventrated diaphragm (which was thin and transparent), followed by “dual-layer sandwich mesh repair”, through an open transthoracic approach. In 2009, Hori et al,15 reported laparoscopic resection of an abundant eventrated hemidiaphragm using endostaplers.
In most recent reports though, diaphragmatic resection is not performed. The redundant part is not resected but is plicated by suturing. When folds are created they lie underneath the plication line. When a redundant central portion is left after suturing, it is usually invaginated underneath the plication sutures, but it can also be left cephalad to it and used to cover it.16 When folds are created, the “classic” direction of plication is the posterior costo-phrenic angle to the costophrenic angle (thus posterolateral to anteromedial,13 but various other directions have been used, including oblique (anterolateral to posteromedial), transverse, or both transverse and anterioposterior.16
Conclusion
Diaphragmatic eventration is a very rare pathology occurring in both adults and children. Management is through clinical and radiological diagnosis including appropriate surgical treatment either by open or close technique. Our index patient benefitted from open approach by virtue of the low-income setting devoid of cutting edge technology for minimal access technique.
References
- Flageoleh H. Central hypoventilation and diaphragmatic eventration: diagnosis and management. SeminPediatrSurg. 2003;12(1):38–45.
- Zhao S , Pan Z , Li Y, An Y, Zhao L, Xin Jin X et al Surgical treatment of 125 cases of congenital diaphragmatic eventration in a single institution. BMC Surgery 2020; 20(270): 1-6.
- Groth SS, Andrade RS. Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg 2010;89:S2146-50
- Aikaterini N, Andreas M, Paul Z, Nikolaos M, Aikaterini S, Nikolaos K, et al .Video assisted thoracoscopic plication of the left hemidiaphragm in symptomatic eventration in adulthood. J Thorac Dis 2012;4(S1):6-16. DOI: 10.3978/j.issn.2072-1439.2012.s00.
- Christensen P. Eventration of the Diaphragm. Thorax 1959; 14: 311.
- Gazala S, Hunt I, Bédard EL. Diaphragmatic plication offers functional improvement in dyspnoea and better pulmonary function with low morbidity. Interact Cardiovasc Thorac Surg 2012;15:505-8.
- Le Pimpec-Barthes F, Brian E, Vlas C, Gonzalez-Bermejo J, Bagan P, Badia A, Riquet M, Similowski T. [Surgical treatment of diaphragmatic eventrations andparalyses. Rev Mal Respir. 2010 Jun;27(6):565-78.
- Groth SS, Andrade RS. Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg. 2010 Jun;89(6):S2146-50
- De Vries TS, Koens BL, Vos A. Surgical treatment of diaphragmatic eventration caused by phrenic nerve injury in the newborn. J Pediatr Surg 1998;33:602–5.
- Stone KS, Brown JW, Canal DF, et al. Long-term fate of the diaphragm surgically plicated during infancy and early childhood. Ann Thorac Surg 1987;44:62–5.
- Haller JA, Pickard LR, Tepes JJ, et al. Management of diaphragmatic paralysis in infants with special emphasis on selection of patients for operative plication. J Pediatr Surg 1979;14:779-85.
- Thomas TV Congenital eventration of the diaphragm. Ann Thorac Surg 1970;10:180-92.
- Leo F, Girotti P, Tavecchio L, et al. Anterior diaphragmatic plication in mediastinal surgery: the “reefing the mainsail” technique. Ann Thorac Surg 2010;90:2065-7.
- Di Giorgio A, Cardini CL, Sammartino P, et al. Dual-layer sandwich mesh repair in the treatment of major diaphragmatic eventration in an adult. J Thorac Cardiovasc Surg 2006;132:187-9.
- Hori T, Masuda K, Taniguchi K, et al. Transperitoneal laparoscopic surgery using endostaplers for adult unilateral diaphragmatic eventration. Surg Laparosc Endosc Percutan Tech 2009;19:e46-50.
- Stolk J, Versteegh MI. Long-term effect of bilateral plication of the diaphragm. Chest 2000;117:786-9.