Lung metastasis at initial presentation in a 3 year old girl with stage IV Wilms tumor
Usoro U U1, Akinola R A2, Ekpe E E3, Fasan-Odunsi A2
Abstract
Background: Wilms tumor (nephroblastoma) is a malignant pediatric tumor that arises from mesodermal embryological precursors of renal parenchyma. Lung metastasis as the first presentation of nephroblastoma occurs in 20% of cases. However, haematogenous spread to the lungs or to regional lymphnodes from a previously diagnosed nephroblastoma occurs in of 85% of cases.
Case report: A 3-year old girl presented at children emergency unit with cough and weight loss. Examination revealed an emaciated child, with a huge, right sided abdominal mass. Abdominal ultrasound scan showed a heterogenous, spherical mass with irregular anechoic areas and echogenic solid areas in kidney. Chest radiograph revealed bilateral cannon ball opacities. A diagnosis of nephroblastoma with lung metastasis was made. She underwent combined therapy but relapsed one year later.
Keywords: Wilm’s tumour, nephrobastoma, lung metastasis, stage IV
Introduction
Wilms tumor (nephroblastoma) is a malignant pediatric tumor that arises from mesodermal embryological precursors of renal parenchyma (metanephros)2 and occurs in 1 in 10,000 live births.1 It develops within the kidney or in the retroperitoneum constituting 90% of tumors in childhood with over 85% of cases2 occurring in early childhood (1 to 11years) peaking at age 3 to 4 years. It makes up 6% of all childhood cancers being the 3rd most common malignancy in childhood after leukemia and brain tumors.1 It is also the 3rd commonest renal mass in childhood (about 87%) after hydronephrosis and multicystic dysplastic kidney1. It is uncommon over 5years of age and rare in neonates and young adults.1 Nephroblastoma is bilateral in 4-13% of patients with no gender predilection though it occurs later in girls and is more common in people of African descent1. Wilms tumor exhibits the rule of 10’s (10%); 10% having unfavorable histology, 10% bilateral, 10% vascular invasion, 10% calcifications, 10% multifocal and 10% pulmonary metastasis.1
Its various associations1,2,3 include isolated abnormalities such as hemi-hypertrophy, sporadic aniridia (severe hypoplasia of iris) 33%, cryptorchidism 2.8%, hypospadias 1.8% and renal fusion. It is primarily familial in 1 – 2% of cases. Nephroblastoma in the 2-24months age group, is usually associated with overgrowth syndromes such as Beckwith-Wiedemann syndrome and Perlman syndrome.2 It is also associated with non-overgrowth syndromes such as WAGR (Wilms tumor, Aniridia, Genitourinary abnormality and mental Retardation) syndrome and Drash syndrome (male pseudo-hermaphroditism and progressive glomerulonephritis).1
Risk factor for metastasis include nephroblastosis which refers to diffuse or multifocal involvement of the kidneys with nephrogenic rests (persistent metanephric blastema that persist beyond 36 weeks of gestation and have potential for malignant transformation into Wilms tumor in 30-40% of cases). Also nephrogenic rests are found in 99% of bilateral Wilms tumor.4,8
Wilms tumor precipitates adrenocortical tumor and hepatoblastoma, and may cause acquired vonWillebrand disease in 8% cases3 displacing adjacent structures due to mass effect. It spreads directly into renal vein or inferior vena cava, into the liver in 7% and very rarely to the bone late in the disease. Lung metastasis as the first presentation of nephroblastoma occurs in 20% of cases. However, haematogenous spread to the lungs or to regional lymph nodes from a previously diagnosed nephroblastoma occurs in of 85% of cases.
The rarity of lung metastasis as the first presentation in nephroblastoma and its antecedent mortality led to the interest of this case.
Case report
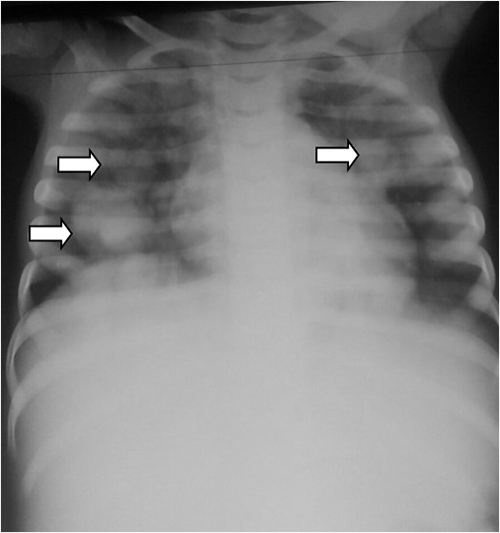
Figure 1: Chest radiograph showing cannon ball opacities (white arrows) in both lung fields
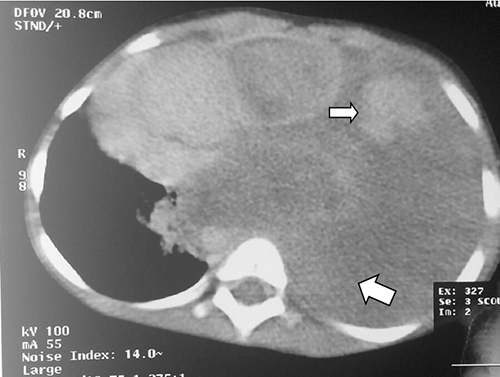
Figure 2: Axial contrast enhanced computed tomography of the chest showing massive left pleural effusion ( large white arrow ) and cannon ball densites (small white arrow) with mediastinal shift to the contralateral side and normal right lung field
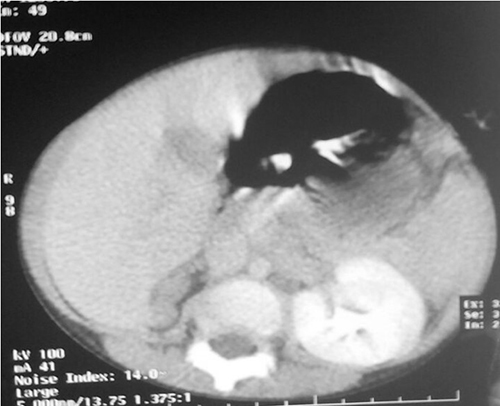
Figure 3: Axial contrast enhanced computed tomography of the abdomen showing absence of right kidney from the renal bed and a well enhanced left kidney
A 3year old girl presented at accident and emergency department with persistent cough and loss of weight. Examination revealed an emaciated and cyanosed little girl in respiratory distress with generalized coarse crepitation. She had a huge, right sided, firm, abdominal mass and an abdominal ultrasound scan showed a heterogeneous, spherical mass with irregular anechoic areas (cystic due to necrosis or hemorrhage) and echogenic solid areas. She also had a chest radiograph which revealed cannon ball opacities (figure 1). A diagnosis of nephroblastoma with lung metastasis was made.
She was then planned for pre-surgical neo-adjuvant chemotherapy, right nephrectomy and post-surgical chemotherapy. Histological examination of the nephrectomy specimen confirmed the diagnosis of nephroblastoma. She was then monitored with 3-monthly serial chest radiographs for about one year during which the chest lesions reduced in size and number before she was lost to follow up.
A year later she presented again, this time with progressive breathlessness. Examination this time revealed a wasted, little girl in respiratory distress and respiratory rate of 64cycles/min. She was also moderately pale. The trachea was deviated to the right. There were stony dull percussion notes and absent breath sounds in the left lung field and transmitted sounds in the right lung field.
Chest radiograph showed a left pleural effusion with mediastinal shift to the right and crowding of the pulmonary vessels raising a suspicion of a probable malignant effusion. Ultrasound scan of the chest showed hyperechoic small round masses within the pleural effusion. Computerized tomography (CT) axial image of the chest/upper abdomen revealed a large, left pleural effusion with a density of 10-52.3 Hounsfield Unit (denser than water). Hyperdense foci were noted in the effusion. There was contra-lateral displacement of mediastinum to right hemithorax (figure 2). The visualized right lung field was clear. The bony thorax was within normal limits. The liver was normal and the right kidney was not visualized (figure 3). A diagnosis of left pleural effusion due to metastatic lung disease was made. A 3-D volume rendering confirmed the above findings. Thoracentesis was negative for clotted blood. She was given more courses of chemotherapy but continued to deteriorate and eventually died.
Discussion
Wilms tumor typically occurs in children and rarely in adults. Most cases are sporadic but some occur in association with overgrowth syndromes such as Beckwith-Wiedemann syndrome and Perlman syndrome.2 It is also associated with non-overgrowth syndromes such as WAGR syndrome and Drash syndrome1 and isolated abnormalities such as hemihypertrophy, sporadic aniridia, cryptorchidism, hypospadius and renal fusion. Most patients are otherwise normal, though patients with Drash syndrome have bilateral or multiple lesions. Rupture puts patient at risk of bleeding and peritoneal spread.
Nephroblastoma occurs in 90% of cases in childhood with over 85% of cases2 involving early childhood of ages 1to 11years with a peak age of 3 to 4 years. The case presented is a 3 year old female who did not have any risk or no known associated factors.
Haematogenous spread to the lungs or to regional lymph-nodes in occurs in 85% of cases while 20% present as lung metastasis at first presentation. This case had pulmonary metastasis at first presentation either due to late presentation by ignorant parents as often seen in our environment or she fell into the above 20% cases.
In children, Wilms tumor, Ewings sarcoma, neuroblastoma and osteosarcoma send secondaries to the lungs as seen in this case. Lymphatic spread is less common and endo-bronchial spread is rare occurring in alveolar cell carcinoma. Primary tumor sites for pulmonary metastasis in adult maybe breast, thyroid and skeleton but urogenital system makes up about 80%. Gastrointestinal and testicular tumors also metastasize to the lungs.
Clinical presentation of nephroblastoma is typically with an asymptomatic, painless, upper quadrant abdominal mass. Metastatic lung disease maybe asymptomatic or present with cough, haemoptysis, chest pain, breathlessness, weakness and weight loss. This case initially had cough and weight loss but presented 2 years post neoadjuvant chemotherapy and nephrectomy with cough, breathlessness and respiratory distress.
Imaging include computerized tomography (CT), chest radiograph, CT guided lung needle biopsy. Non-imaging investigations include sputum examination and surgical excision biopsy. Metastases make up 3% of asymptomatic pulmonary nodules while 75% present as multiple, bilateral, symmetrical with basal predominance with the size range from millimeters to centimeters. Well defined lesions were from childhood tumors mentioned above while ill-defined lesions were found in primaries affecting prostate, stomach and breast. The pulmonary nodules may cavitate and result in spontaneous pneumothorax. Calcifications are unusual. Endobronchial affectation are usually segmental.
Chest radiographs usually show coarse, linear, reticulo-nodular basal shadowing with pleural effusion and hilar lymphadenopathy. This patient’s chest radiograph (figure 1) showed cannon ball opacities of varying sizes in the lung (indicative of pulmonary metastasis) with ill-defined areas of consolidation with air-bronchogram pattern in both lung fields (indicative of pneumonia), left pleural effusion with mediastinal shift to the right.
High resolution CT of the chest in lung metastasis show nodular thickening of interlobular septa and thickening of the centrilobular and bronchovascular bundles.3,4 This case presented had left pleural effusion, lung collapse and soft tissue density foci in its lung field (figure 2).
Management of lung metastasis is usually by radiation, laser, chemotherapy, stents or heat probes. The presented case had systemic chemotherapy and nephrectomy after presentation.
Prognosis is about 5 year survival rate for metastatic lung disease. Recurrence of Wilms tumor can occur in the left-over of the nephrectomy kidney. This patient however died about 3 years after presentation.
Summary
A case of metastatic lung disease in a 3 years old girl with stage IV Wilms tumor at first presentation was reported and followed up for a year post chemotherapy and nephrectomy. The role of radiology in diagnosis has been outlined. All cases of Wilms tumour should be investigated at initial presentation for distant metastasis.
References
- Graham SD, Keane TE, Gleun’s Urologic surgery. Lippincott Williams and Wilkins 2009; ISBN: 0781791413.
- Geller E, Smergel EM, Lowry PA. Renal neoplasm of Childhood. Radiologic Clinics of North America 1997;35:1391 -1413.
- Lowe LH, Isuani BH, Heller RM. Pediatric renal masses, Wilms tumor and beyond. Radiographics 2000; 20: 1585 – 1603.
- Chilles C, Ravin CE. Intrathoracic metastasis from extrathoracic malignancy radiographic approach to patient evaluation. Radiologic Clinics of North America, 1985; 23:427-438
- Sutton D. Textbook of Radiology Imaging Churchill Livingstone 7th Edition, Elsevier. 2004; 2: 961.
- Coppage L, Shaw C, Curtis AM. Metastatic disease to the chest in patients with extrathoracic malignancy. Journal of Thoracic Imaging. 1987;2(4):24-37.
- Vourtsi A, Gouliamos A, Moulopoulos L. CT. appearance of solitary and multiple cystic and cavitatory lung lesions. Eur. Radiol, 2001;11:612-622.
- Rankin SC, Webb JA, Reznek RH. Spiral CT in diagnosis of renal masses. British Journal of Urology Imaging 2000; 86:48-57