Chromophobe: A rare histological variant of renal cell carcinoma
Adekunle A A1, Idowu N A2, Rasheed M W3, Aderibigbe O A1, Olatide O O4
Abstract
Background: Renal cell carcinoma (RCC) is an adenocarcima of the kidney. It is a highly heterogeneous disease with sixteen known subtypes out of which five predominate. The most common subtype is clear cell RCC which accounts for 75-85%. The chromophobe subtype of RCC is rarely seen. It is reported to constitute 4-5% of RCC subtype. This study was aimed at reporting the first case of chromophobe renal cell carcinoma in our community a rare histological subtype of RCC which was discovered incidentally. Our objective was to describe the clinical, histological and immunohistochemical evaluation of a case of chromophobe RCC.
Case report: We present a 60-year-old man who was seen at the surgical outpatient department of our center with a request for general health check-up. He had basic laboratory and imaging investigations. These were essentially normal except for an incidental finding of a left renal mass on abdominopelvic sonogram. Computed tomography urography showed a suspected clinically localized malignant left renal mass. Histological and immunohistochemistry are consistent with a chromophobe RCC. His clinic follow-up remains unremarkable.
Conclusion: We reported the clinical, histological and immunohistochemical evaluation of a rare case of chromophobe RCC. This was discovered incidentally following general health check. He had open radical nephrectomy and clinic follow-up 1 year after discharge has been uneventful.
Keywords: Renal cell carcinoma, chromophobe, immunohistochemistry.
Introduction
Renal cell carcinoma (RCC) is an adenocarcinoma of the kidney. It is the most lethal genitourinary malignancy.1 It accounts for 2-3% of cancer disease.2 It is sporadic in 98% of cases.3 Approximately 2-4% of RCC is hereditary.4 Family history of RCC, hypertension, cigarette smoking and obesity have been to a large extent consistently linked with the development of RCC.5 Renal cell carcinoma is considered to be an heterogeneous disease with sixteen known histological subtypesout of which five predominate.6 The commonest histological subtype is clear cell RCC which accounts for 75-80% of cases. Papillary RCC accounts for 10-15% of cases. Chromophobe accounts 4-5% and collecting duc RCC accounts for 1-2%.7 Others are unclassified. Chromophobe RCC like some other histological types may be an incidental finding following investigation for unrelated clinical conditions. Symptomatic patients may present with hematuria, loin pain or loin mass in advanced cases. This is often referred to as too late triad.8 The diagnosis of RCC is established by cross-sectional imaging. This is confirmed by histopathological analysis of the specimen following surgical removal of the tumor. The role of pre-operative renal biopsy is controversial. The diagnosis of chromophobe variant of RCC is challenging due to the similarities of its clinical and histomorphological properties with some other histological subtypes of RCC such as renal oncocytoma, and oncocytic variant of papillary RCC.9 The role of immunohistochemistry cannot be over emphasized. The treatment option for RCC depends on the clinical stage of disease. The options that are commonly being explored are active surveillance, partial nephrectomy, radical nephrectomy, radiofrequency ablation and cryoablative surgery. Most cases of chromophobe RCC are low grade and usually confined to the kidney. This may be responsible for its good prognosis compared to other histological subtypes.
Case presentation and management
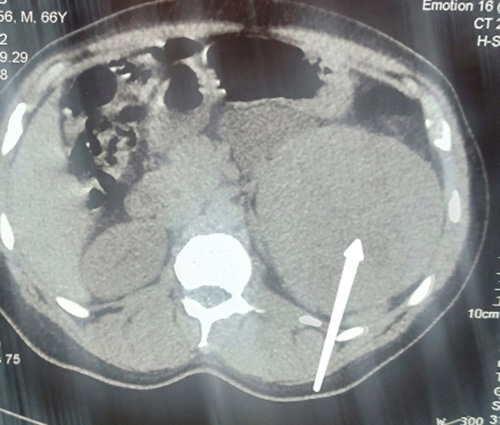
Figure 1a
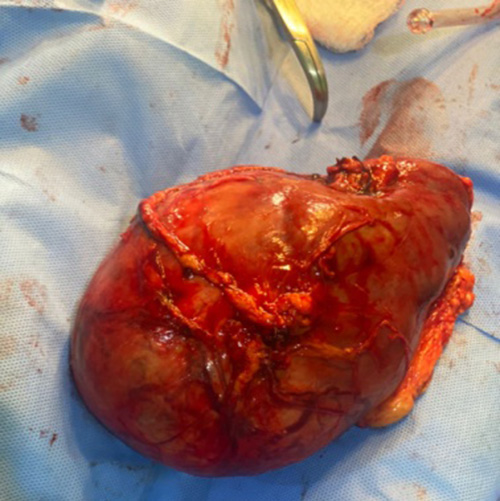
Figure 1b

Figure 1c
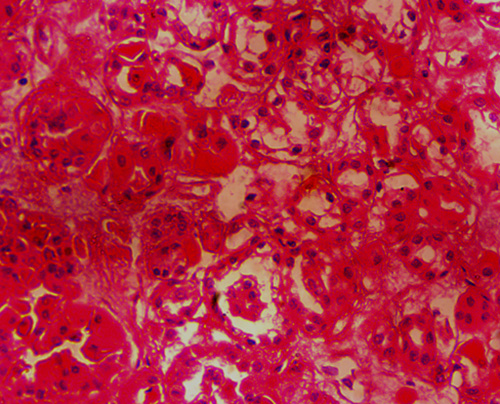
Figure 2a
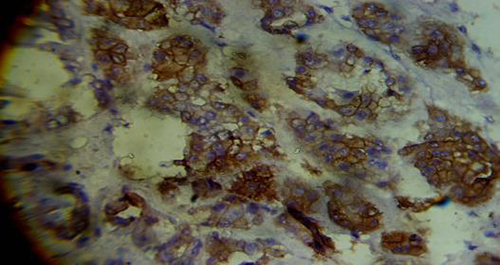
Figure 2b
Legend to figures 1a, b, c, 2a, b.
Figure 1a: Computed tomography film showing left renal mass as indicated by the left arrow.
Figure 1b: Gross clinical photograph of the mass.
Figure 1c: Cut section which is brownish in appearance.
2. The cut section was as shown in figure 1c. The immunohistochemistry was positive for CK7 (Figure 2a and 2b).
Figure 2a: Histological appearance of the cHRCC with tumour cells showing eosinophilic cytoplasm.
Figure 2b: Histological section shows immunoreactivity of CK7 with membranous and cytoplasmic staining in 70% of the tumour cells.
A 60year old man presented at the surgical outpatient department of our centre with a request for general health check up. He had no history of lower urinary tract symptoms, flank pain, flank mass or hematuria. He neither takes alcohol nor smoke cigarette. He had no history of exposure to urothelia carcinogen and there was no family history of similar problem. He is a known patient with hypertension on oral amlodipine and hydrochlorothiazide but not diabetic. Clinical examination showed body mass index of 28kg/m2, otherwise unremarkable. He had basic laboratory and imaging investigation for general health check–up. All the investigations were essentially normal except for abdominopelvic sonogram which showed a left renal mass. He had computed tomography urography for further evaluation and a suspected malignant left renal tumor was seen measuring 14.57 by 11.8 by 11.31cm (Figure 1a). The contralateral kidney was essentially normal (figure 1a). He had left radical nephrectomy. Intra-operatively, the tumour was grossly seen confined to the kidney (figure 1b). There was no involvement of the Gerota’s fascia, renal vein or vena-cava. It did not involve any viscera. There was no ascites. Post-operative days were uneventful. Periodic abdominopelvic and chest imaging have been unremarkable. The histopathological analysis of the tumor showed chromophobe RCC, T2 NO MO.
Macroscopically, the specimen weighs 1kg and measures 15x12x10cm. Cut surface shows a well-circumscribed mass which is brownish in appearance (Figure 1b and 1c). Histological section shows renal tissue composed of epithelial neoplasm made up of proliferating pleomorphic malignant cells with hyperchromatic to vesicular nuclei, prominent nucleoli and clear to varying amounts of eosinophilic cytoplasm. The tumor cells are disposing in tubules, cysts and pseudo-papillary formation in areas. They are seen as cords and nests also. There are focal areas of necrosis (Figure2a). Pathological diagnosis of renal cell carcinoma, most probably chromophobic, was made. Immunohistological section shows immunoreactivity of CK7 with membranous and cytoplasmic staining in 70% of the tumour cells (Figure 2c)
Discussion
Patients with chromophobe renal cell carcinoma chRCC are mostly younger female and present at an earlier stage. In this case, the patient is male and older. Additionally, the clinical course of chRCC is less aggressive as compared to chromophobe renal cell carcinoma ccRCC. Most patients are asymptomatic at diagnosis, especially those detected as an incidental renal mass on imaging as demonstrated in this case study. This is similar to the report from other series that showed that 58% cases of chromophobe RCC are asymptomatic and may be incidental. The classic triad of flank pain, hematuria, and palpable abdominal mass are rarely seen, if present, is suggestive of locally advanced or metastatic disease. This may be due to its low-grade morphology. Hypertension and abnormal body mass index were the likely risk factors noted in our patient. Both hypertension and obesity have been consistently linked with the development of RCC.5
Imaging study is vital in pre-operative diagnosis of RCC with Computed tomography (CT scan) being the most common imaging modality for diagnosis and staging. Radiologically, it is not possible to differentiate the RCC subtypes with certainty. Histology is a gold standard for diagnosis. It is required that routine histological stain should be complemented with immunohistochemistry as well as genetic study to differentiate subtypes of RCC and to differentiate oncocytoma from chromophobe RCC.
Grossly, chRCC is a well-circumscribed unencapsulated mass with solid, homogeneous, and pale tan to dark brown cut surfaces as seen in this case. Gross hemorrhage, necrosis, cystic changes, and a central scar may be seen. Microscopy appearance shows varying proportions of pale cells and eosinophilic cells. The pale cells are large and polygonal with abundant flocculent cytoplasm. The eosinophilic cells are smaller and have granular eosinophilic cytoplasm. The typical chRCC is composed of these two cell types. The tumour cells may dispose in tubular, cystic or papillary formations. ChRCC is not assigned a grade due to its inherent nuclear atypia. Sarcomatoid changes are infrequent (2–8 % of cases) and often show an abrupt transition from the epithelial components.10 Sarcomatoid changes are a sign of aggressive behavior and poor prognosis. Notably, we did not see sarcomatoid differentiation in this case. Absence of sarcomatoid change connotes good prognosis as demonstrated in this case.
Immunohistochemically, the tumor cells in chRCC are diffusely positive for CD117 (C-Kit) and CK7. Renal oncocytoma (RO) has histological similarities with chRCC especially with eosinophilic variant where IHC can help resolve the diagnostic dilemma.11 ChRCC has diffuse, strong positive Ck7 with membranous and cytoplasmic staining in 70% of the tumour cells, whereas it is usually negative for RO as demonstrated in this case study.12
Our patient did well following radical nephrectomy. Radical nephrectomy is the treatment of choice for patient with clinically localized renal cell carcinoma.
Conclusion
We reported the clinical, histological and immunohistochemical evaluation of a rare case of chromophobe RCC. This was discovered incidentally following general health check. He had radical nephrectomy and clinic follow-up 1 year after discharge has been uneventful.
References
- Banumathy G, Cairns P. Signaling pathways in renal cell carcinoma. Cancer biology & therapy. 2010;10(7):658-64.
- Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World journal of radiology. 2016;8(5):484.
- Audenet F, Yates DR, Cancel Tassin G, Cussenot O, Rouprêt M. Genetic pathways involved in carcinogenesis of clear cell renal cell carcinoma: genomics towards personalized medicine. BJU international. 2012;109(12):1864-70.
- Menko FH, Maher ER. Diagnosis and management of hereditary renal cell cancer. Rare Hereditary Cancers: Diagnosis and Management. 2016:85-104.
- Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, et al. Risk factors for renal cell carcinoma in the VITAL study. The Journal of urology. 2013;190(5):1657-61.
- Gürel S, Narra V, Elsayes KM, Siegel CL, Chen ZE, Brown JJ. Subtypes of renal cell carcinoma: MRI and pathological features. Diagnostic and interventional radiology. 2013;19(4):304.
- Yin X, Wang J, Zhang J. Identification of biomarkers of chromophobe renal cell carcinoma by weighted gene co-expression network analysis. Cancer cell international. 2018;18(1):1-12.
- Campbell SC. Commentary RE: Increased Incidence of Serendipitously Discovered Renal Cell Carcinoma. Urology. 2020;145:333.
- Alaghehbandan R, Przybycin CG, Verkarre V, Mehra R. Chromophobe renal cell carcinoma: novel molecular insights and clinicopathologic updates. Asian Journal of Urology. 2021.
- Cao W, Chen H-D, Yu Y-W, Li N, Chen W-Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chinese medical journal. 2021;134(07):783-91.
- Ng KL, Rajandram R, Morais C, Yap NY, Samaratunga H, Gobe GC, et al. Differentiation of oncocytoma from chromophobe renal cell carcinoma (RCC): can novel molecular biomarkers help solve an old problem? Journal of Clinical Pathology. 2014;67(2):97-104.
- Casuscelli J, Becerra MF, Seier K, Manley BJ, Benfante N, Redzematovic A, et al. Chromophobe renal cell carcinoma: results from a large single-institution series. Clinical genitourinary cancer. 2019;17(5):373-9. e4.