Survival following recurrent sustained ventricular tachycardia in a low-resource setting: Challenges of management
Shogade TT1, Bassey EA1, Udosen AU1, Ekuma IA1, Bassey AE1, Akpabio AA1, Umoh IO1, Sogade FO2
Abstract
Sustained ventricular tachycardia is a life-threatening emergency contributing to cardiac mortality worldwide. Antiarrhythmic medications, Implantable cardioverter defibrillator and catheter ablation are the cornerstones for its management. Unfortunately, they are not accessible and/or affordable to patients seen in our setting.
42year old obese, retroviral positive, known ischemic heart disease patient, was admitted twice due to recurrent monomorphic ventricular tachycardia and successfully cardioverted with amiodarone infusion and automated external defibrillator respectively, He could not have coronary angiography and electrophysiology studies done due to unavailability and cost. Optimal management of ventricular tachycardia continues to be a challenge for cardiologists in developing countries.
Keywords: Ventricular tachycardia, electrocardiogram, defibrillator, ischemic heart disease, rhythm disorder.
Introduction
Ventricular tachycardia (VT) is a life-threatening medical emergency especially when sustained. In Sub-Saharan Africa (SSA), sudden cardiac death (SCD) often goes undiagnosed with such occurrences attributed to supernatural causes.1 Although the burden of VT is not known in Sub-Sahara Africa, ventricular tachycardia has been reported to account for SCD in this clime.1
The management of VT is very challenging in low and middle-income country (LMIC) with important diagnostic and therapeutic devices largely unavailable leading to frequent mortality.2 There is need for immediate management of sustained VT with defibrillation and subsequent antiarrhythmic drugs, while coronary revascularization is needed for long-term treatment and prevention of VT when due to ischemic heart disease.3
Other chronic medical conditions which are risk factors for arrrhythmia include; hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, obstructive sleep apnea, asthma, chronic obstructive pulmonary disease, hyperthyroidism, hypothyroidism, depression, venous thromboembolism, peripheral arterial disease, stroke and coronary artery disease. This case highlights the challenges faced in the diagnosis and management of sustained VT in SSA.
Case report
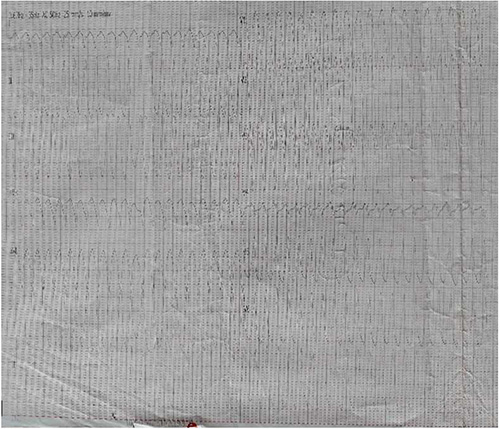
Figure 1: ECG showing monomorphic ventricular tachycardia (22/07/2020)
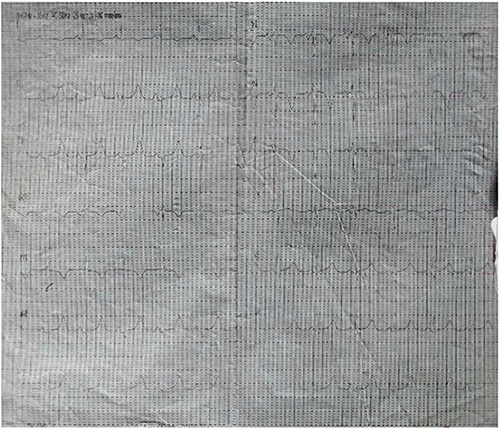
Figure 2: ECG after Amiodarone infusion showing sinus rhythm, old inferior myocardial and antero-septal myocardial ischemia (23/07/2020)
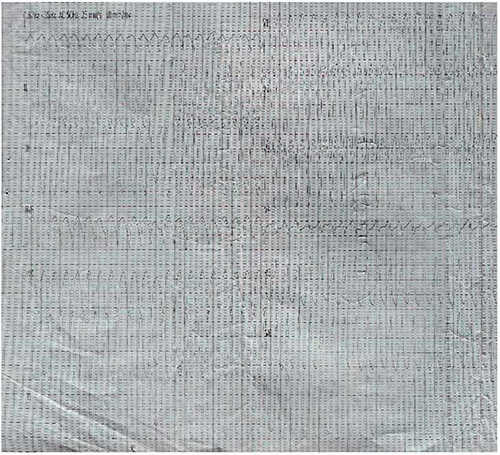
Figure 3: ECG showing monomorphic ventricular tachycardia (23/12/2020)
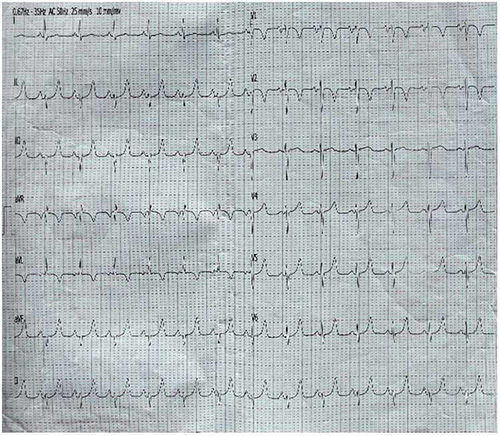
Figure 4: ECG after cardioversion showing sinus rhythm and septal myocardial ischemia
A 42-year-old male with HIV treated with combination anti-retroviral therapy (cART). He was regular with his medications consisting of tenofovir, lamivudine and dolutegravir with good viral/immunological response (viral load <40copies (15/04/2019), CD4 count 433cells/mm3 (2/3/2020)). He presented acutely in clinic 22/07/2020 with a 3-day history of dull retrosternal chest pain associated with nausea, vomiting and diaphoresis. This was followed by frequent palpitations, dyspnea at rest, orthopnea and paroxysmal nocturnal dyspnea. He had presented 2 months earlier with pleuritic chest pains and was treated for pericarditis.
Examination revealed an obese male (BMI 40.2kg/m2), tachypneic (respiratory rate of 44 cycles per minute), with oxygen saturation 99% on room air, pulse rate 42beats/minute, completely irregular, heart rate 125beats/minute, blood pressure 100/80mmHg and first, second and fourth heart sounds audible. His electrocardiogram (ECG) showed monomorphic ventricular tachycardia (Figure 1). He was immediately transferred to the ward where he was given intranasal oxygen, oral bisoprolol 5mg daily, aspirin 150mg daily and rabeprazole 20mg twice daily.
Eight hours later, he was started on slow intravenous infusion (IV) of 150mg amiodarone in 17mls of 5% glucose water over 15minutes as a stat dose, followed by a slower 150mg IV infusion of amiodarone in 200mls of 5% glucose water to run over 6hours. Other drugs given post-infusion were rosuvastatin 40mg daily, subcutaneous enoxaparin 40mg 12hourly, oral amiodarone 200mg twice daily after the infusion. Patient’s infusion was started late because the drug was sourced outside the hospital. As there was no functional automated external defribillator (AED) then in the hospital, he couldn’t be defibrillated by AED.
Serum CK-MB (2.33ng/ml (<5.1) and high-sensitive Troponin I 29.8ng/ml (<100) were normal both done 5days post admission due to paucity of funds. Echocardiography showed asymmetrical septal hypertrophy with normal left ventricular (LV) systolic function (ejection fraction of 72%) and grade two LV diastolic dysfunction (E/A ratio 1.6, E/e’ 8.5, dilated left atrium: 4.7cm).
Daily ECG done after admission showed initial VT followed by features of old inferior myocardial infarction and anteroseptal myocardial ischemia up to 3 days post-admission (figure 1b). Blood urea and creatinine were elevated 23.1mmol/l (2.1-7.1) and 372 mmol/l (53-115) respectively, but thyroid function test, fasting lipid profile and fasting blood glucose were normal. Patient could not do coronary angiography nor electrophysiology study due to non-availability in our center and lack of funds.
He had sustained improvement with cardiovascular stability and was discharged home after 5 days on his oral medications (amiodarone 200mg twice daily, aspirin-clopidogrel 75/75mg daily, bisoprolol 5mg daily, rosuvastatin 40mg daily, rabeprazole 20mg daily and cART). After sometime, he defaulted from clinic visit and was not taking his drugs except the cART due to lack of funds.
The patient presented 4 months later 23/12/2020 at our clinic with recurrent sudden sharp central chest pain started while having his bath, and palpitation, breathlessness, vomiting and diaphoresis of 3 days duration. He was restless, had bilateral leg edema, his pulse rate was 140 beats/minutes, small volume, and blood pressure 80/50mmHg, he was admitted initial ECG showed VT which converted back to sinus rhythm immediately after defribillation with a single 200 joules direct current shock using an AED owned by the hospital. His blood pressure and pulse rate became 90/60mmHg and 76beats/minute respectively.
Patient was discharged home after 8th days still in sinus rhythm on his medications, counseled to do coronary angiography and come for follow up after two weeks, he spent longer period on admission due to delayed in carrying out requested investigations. Patient was last seen in our clinic 55days post discharge, he was stable and latest ECG still showed sinus rhythm (figure 1c).
Discussion
We reported a 42 year old man who survived recurrent VT. Ventricular tachycardia is life-threatening and if not treated promptly it can progress to ventricular fibrillation and eventually death.4
Though VT commonly occur in structural heart disease which could be ischemic or non-ischemic.5,6 It can also arise from structurally normal heart such as Brugada syndrome, Long QT syndrome and Duchenne muscular dystrophy.7,8
Ventricular tachyarrhythmias usually occur as a result of enhanced automaticity, triggered activity by early or late after-depolarizations, or reentry. In acute myocardial infarction, the ischemia results in an increased concentration of extracellular potassium, which causes partial depolarization of the resting membrane potential. This creates injury currents between the infarcted tissue and healthy myocardium that may trigger spontaneous activity.9 Frequent manifestation of VT in IHD could also be a result of reentrant arrhythmia arising from fixed substrate composed of scarred and residual viable myocardium
Our index patient was at high risk of VT as a consequence of his background chronic medical conditions, which are obesity, ischemic heart disease, HIV infection and renal impairment. People living with HIV/AIDS (PLHA) have higher likelihood of developing VT due to accelerated atherosclerosis, or cardiac dilation as a sequalae of myocarditis, infective endocarditis or pericarditis from the virus itself and other opportunistic infections.10
Polypharmacy in the treatment of HIV include drugs such as evafirenz, ganciclovir, amphotericin B, antifungals, macrolide, pentamidine and trimethoprim sulphamethoxazole which can lead to unusually prolonged repolarization, electrolyte abnormalities or conduction slowing. Non-sustained VT has been reported in 15-23% of PLHA.11
Ischemic heart disease has been on the increase in Sub-Sahara Africa (SSA) as a result of increasingly westernized lifestyles and awareness among cardiologists.12 Till date, diagnostic and therapeutic facilities are largely inadequate, thus making emergency treatment and long term management difficult though not totally inaccessible.
This was the case in this patient, who could not get infusion amiodarone immediately he presented the first time, because it had to be bought outside the hospital from a private vendor. Also challenging is the fact that, he was not able to have coronary angiography, implantable cardioverter defribillator (ICD) implanted, electrophysiology study nor ablation due to lack of funds, because majority of patients in our setting pay for health services out of pocket.
However, despite relative paucity in the definitive management of VT in this clime, Edafe et al13 reported challenges encountered in the management of three patients with ventricular tachycardia in South-South Nigeria, who were initially managed conservatively due to lack of funds and later had ICD implanted.
Outcome of VT, depends on the promptness of diagnosis and available resources, this patient survived two episodes of VT, similarly Ali et al reported a 55 year old smoker who survived unstable sustained VT following defibrillation, IV lignocaine and oral amiodarone administration.14 Hummadi et al also reported a case of artesunate induced ventricular tachycardia in a patient with sickle cell disease which was aborted with intravenous amiodarone infusion.15
Unlike the case reported by Nkoke et al4 which resulted in a fatality 30 minutes after emergency room arrival because a defibrillator was not immediately available in the hospital and was administered six tablets of 200mg amiodarone, oxygen by nasal cannula and intravenous fluids. This is often the situation in developing countries facing the twin burden of infections and lifestyle diseases thus further stretching already limited resources. Additionally, a lack of awareness and adequate insurance coupled with treatment delay at different levels leads to poor outcomes.
Likewise, Jingi et al reported the case of a 30-year old woman who presented in a tertiary health centre in Sub-Saharan Africa in shock and a diagnosis of VT was made. However could only had oral propranolol and amiodarone before referral to facility abroad where electrophysiology studies showed two inducible foci of VT and one was successfully ablated.1
The initial management of patient with VT depends on patient’s stability. Ventricular tachycardia at rate below 150 beats/min is usually well tolerated whereas at rate above 200 beats/min is frequently unstable. This is in keeping with our patient who had unstable ventricular tachycardia of 254 beats per minute.
For clinically stable patient antiarrhythmic drugs (AADs) are the mainstay of treatment, in unstable sustained VT, the first line is stabilization, by electrical cardioversion to sinus rhythm using automated external defibrillator (AED) follow by AADs. The intravenous AADs available are procainamide, lidocaine, amiodarone and beta-blockers such as metoprolol, sotalol and esmolol.
Their availabilities are usually not guaranteed, even IV lidocaine is usually in local anesthetics formulation. Procainamide is contraindicated if there is left ventricular dysfunction because it can exacerbate heart failure, amiodarone should not be first line if LV function is normal because its effect on myocardial conduction and refractoriness are gradual, combine with multiple side effects such as thyroid dysfunction, lungs fibrosis, liver fibrosis and skin pigmentation, etc.16,17
The long-term management of VT is influenced by the presence or absence of structural heart disease (SHD). ICD is indicated in almost all patients with ventricular arrhythmias and SHD. While the ICD is effective in preventing SCD due to VT or ventricular fibrillation in patients with heart failure, AADs with ICD programming are more effective in treatment and secondary prophylaxis of patients with VT and SHD.
Beta blocker or calcium channel blocker can be used in long term treatment of idiopathic VT with good safety profile. Ranolazine, an inhibitor of the late inward sodium current initially used for ischemic heart disease has been found to be effective in VT suppression in patient with ICD implantation having complaints of recurrent ICD shocks that is not responding favorably to other antiarrhythmic drugs.18
With the development of electroanatomic mapping, advances in ablation technology and the development of epicardial approaches, ablation has become an effective intervention in an increasingly broad range of VT etiologies, and is now deployed increasingly early in the management of recurrent VT.19 Catheter ablation have been shown to prevent VT recurrences which also favorably affect survival in patients with ventricular tachycardia.20
In arrhythmogenic inflammatory cardiomyopathy, immunosuppressive therapy may be effective in controlling VT and preventing recurrence either as monotherapy or in conjunction with ablation.6
In our case, the choice of IV amiodarone for the first episode was due to his background heart condition and availability. Fortunately for the second presentation, an AED was readily available and he improved after defibrillation. Insertion of ICD and ablation of VT focus was not done because they were not available and costly.
About 18% of SSA countries have no cardiac implantable electronic devices services, management of tachyarrhythmia is mainly noninvasive (via rate controlled strategy) as electrophysiology study and catheter ablation centers is almost non-existent in most countries and implant rates for bradyarrhythmias are more than 200-fold lower than in the Western countries.21
In conclusion, cardiac diseases including VT is on the increase in developing countries, outcomes of patient’s care depend on availability of resources. We report this case to highlight the peculiar challenges face in the management of VT in developing countries, though this patient is lucky to have survived two episodes of sustained VT.
We hereby recommend that our hospitals should be equipped for basic and advanced cardiac life support and at least each of the 6 geopolitical zones should have a dedicated coronary care unit and electrophysiology laboratory.
References
- Jingi AM, Boombhi J, Mfeukeu-kuate L, Hamadou B, Abas A, Menanga A. Diagnostic and therapeutic challenges of sustained ventricular tachycardia revealing cardiac lipoma in sub-Saharan Africa : a case report of a 31-year old woman in Cameroon. J Xiangya Med. 2018;3:41-47. doi:10.21037/jxym.2018.11.03
- Nkoke C, Luchuo EB, Dikoume L. Fatal monomorphic ventricular tachycardia in a semi ‑ urban setting in Cameroon : a case report. BMC Res Notes. 2017;(May):10-13. doi:10.1186/s13104-017-2501-4
- Koplan B, Stevenson W. Ventricular Tachycardia and Sudden Cardiac Death. Mayo Clin Proc. 2009;84(4):289-297.
- John R, Tedro U, Koplan B. Ventricular arrhythmias and sudden cardiac death. Lancet L Engl. 2012;380(2):1520-1529.
- Nwafor CE, Alikor CA. Atypical presentations and other challenges in the management of acute coronary syndrome in developing countries. 2017;11(1):42-44. doi:10.4103/phmj.phmj
- Tung R, Bauer B, Schelbert H et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Hear Rhythm. 2015;12:2488-2498.
- Tang P, Do D, Li A, Boyle N. Team Management Of Ventricular Tachycardia Patients. Arrhythmia Electrophysiol Rev. 2018;7(4):238-246. doi:10.1017/CBO9781107415324.004
- Shogade T., Akpabio A., Markson F., Sogade F. Case Report An Unusual Case of Brugada Syndrome in an 82-Year Old Black Hypertensive. J Integr Cardiol Open Access. 2020;3(3):10-12. doi:10.31487/j.JICOA.2020.03.07
- Heart Rhythm 2019. 2019;(1192568):1192568.
- Tang P, Do D, Li A, Boyle N. Ventricular Tachycardia. Arrhythmia Electrophysiol Rev. 2018;7(4):238-246.
- Das M. Cardia arrhythmias in HIV disease. Cardiovasc Rep Rev. 2002;23:208-212.
- Nkoke C, Luchuo E. Coronary heart disease in Sub-Sahara Africa: Still rare, misdiagnosed,or underdiagnosed? Cardiovasc Diagn Ther. 2016;6(1):64-66.
- Edafe EA, Dodiyi-manuel ST. Implantable cardioverter defibrillators in sub-saharan africa center : Three case series and challenges in management. J Integr Cardiol. 2020;6:1-6. doi:10.15761/JIC.1000286
- Ali SY, Ali MY, Rahman MM, Islam MM. Case Report Ventricular Tachycardia – Life Threatening Cardiac Arrhythmia – A Case Report. Faridpur Med Coll. 2010;5(2):72-73.
- Hummadi A, Mubarki S, Al-qahtani AM. Case Report Cardiac Arrhythmia in a Patient with Sickle Cell Anemia and Falciparum Malaria Treated with Intravenous Artesunate. 2019;2019:1-5.
- DeSouza I, Martindale J, Sinert R. Antidysrhythmic drug therapy for the termination of stable, monomorphic ventricular tachycardia: a systematic review. Emerg Med J. 2015;32:161-167.
- Ortiz M, Martín A, Arribas F et al. Randomised comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Hear J. 2017;38:1329-1335. doi:10.1377/hlthaff.2013.0625
- Bunch T, Mahapatra S, Murdock D et al. Ranolazine reduces ventricular tachycardia burden and ICD shocks in patients with drug-refractory ICD shocks. Pacing Clin Electrophysiol. 2011;34:1600-1606.
- Ortiz M, Martín A, Arribas F et al. Randomised comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Hear J. 2017;38:1329-1335.
- Bella P Della, Baratto F, Tsiachris D, et al. Management of Ventricular Tachycardia in the Setting of a Dedicated Unit for the Treatment of Complex. Circulationtion. 2013;127:1359-1368. doi:10.1161/CIRCULATIONAHA.112.000872
- Yuyun MF, Bonny A, Ng GA, et al. A Systematic Review of the Spectrum of Cardiac Arrhythmias in Sub-Saharan Africa. Glob Heart. 2020;15(1):2-30. doi:10.5334/gh.808.