Adverse drug reactions reporting by community pharmacists in Lagos State
Olugbake OA, Rufus AC, Joda A, Balogun M
Abstract
Background: Spontaneous Adverse Drug Reaction (ADR) reporting is the cornerstone of Pharmacovigilance. It is very important that pharmacists are alert to potential adverse drug reactions and drug interactions. The contribution of community pharmacists towards ADR reporting in four regions of Lagos state, Nigeria was studied.
Objective: The main aim of the study was to assess the knowledge, attitude and behaviour of community pharmacists to ADR and its reporting.
Method: This questionnaire based survey was conducted over a period of four months in 30 community pharmacies each (120 respondents) in four areas of Lagos state (Ikorodu, Victoria Island, Surulere and Ikeja). The developed questionnaire contained sections on the demographic details of the pharmacists, knowledge, perception and evaluation of the pharmacists on ADR and its reporting, and factors influencing ADR reporting. The data obtained was entered unto Microsoft Excel and analysed using frequency and percentage.
Results: Over 96% agreed that the spontaneous reporting of suspected ADRs is necessary for pharmacovigilance. The analysis showed that the pharmacists had good knowledge of ADRs but the reporting rate was only 24.2%. This means that though the pharmacists were knowledgeable about ADRs, they were not putting its reporting into practice due to several reasons.
Conclusion: The knowledge of ADRs and its reporting by community pharmacists in Lagos state needs to be improved upon. Continuous education, training and integration of ADR reporting into the clinical activities of the pharmacists by the Pharmacovigilance centre and other relevant personnel would improve reporting.
Introduction
Modern medicines have changed the way in which diseases are managed and controlled. Most drugs produce several effects, but usually only one effect- the therapeutic effect- is wanted for the treatment of a disorder. The other effects may be regarded as unwanted, whether they are intrinsically harmful or not. For example, certain antihistamines cause drowsiness as well as control the symptoms of allergies.1 When an over-the-counter sleep aid containing an antihistamine is taken, drowsiness is considered a therapeutic effect, but when an antihistamine is taken to control allergy symptoms during the daytime, drowsiness is considered an annoying unwanted effect. Drug interactions can also cause adverse reactions. The prescriber therefore has the herculean task of selecting the best and safest medicine for each individual patient per time.
Paracelsus, sometimes called the father of toxicology, wrote:1 ‘All drugs are poisons, and nothing is without poison; only the dose permits something not to be poisonous.’
This is to say that the substances considered toxic are harmless in small doses, and conversely an ordinarily harmless substance can be deadly if over consumed. This underscores the need for care and vigilance with drug use. It is widely accepted that medicines might produce adverse effect during the course of their therapeutic use.1 While the efficacy of a drug can be quantified with relative ease, the same cannot be said about safety. This is because, the adverse effect of a drug may be uncommon (but very serious) and many patients may be affected or subjected to a potential risk before the relationship with the drug is established.
Due to the complexity of therapies, the aging of the population, and the development in multimorbidity, Adverse Drug Reactions (ADRs) continue to be an issue in modern medicine.
ADRs are described as "an appreciably harmful or unpleasant reaction resulting from an intervention related to the use of a medicinal product; adverse effects typically predict risk from future administration and warrant prevention, or specific treatment, or alteration of the dosage regimen, or withdrawal of the product."2
From the very beginning of the drug development process through the clinical trials, information on adverse events is gathered and reported. Afterward, ongoing post-marketing surveillance activities are carried out to compile comprehensive safety data for all medications. Healthcare systems significantly rely on spontaneous reporting of adverse drug reactions (ADRs) to monitor the safety of medications once they have been licensed and marketed globally.3 These aids regulatory agencies in locating drugs that might have undiscovered safety problems that weren't noticed during clinical studies. Regardless of the practice settings, an increase in adverse drug reactions (ADRs) is a problem for global health that needs to be addressed by all parties. According to reports, adverse medication responses are a major contributor to morbidity and death in people of all ages, account for a sizable portion of hospital admissions, and place a huge financial burden on society and healthcare systems.3
Pharmacovigilance (PVG) as defined by WHO is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem.4 WHO established its programme for International Drug Monitoring in response to the thalidomide disaster in 1961. The aims of PVG are to enhance patient care and patient safety in relation to the use of medicines; and to support public health programmes by providing reliable and balanced information for the effective assessment of the risk-benefit profile of medicines.5
The cornerstone of pharmacovigilance efforts is the spontaneous ADR reporting system, which is a worldwide phenomenon. Therefore, healthcare professionals in all capacities play a critical role in the pharmacovigilance system, and as such, they need a great deal of knowledge and skill in the area of medication safety, particularly for the early recognition, detection, management, and reporting of ADRs.6 Nevertheless, studies show that a significant amount of ADRs are not reported by healthcare personnel, particularly in poor nations, for a variety of reasons, including a lack of awareness and expertise of pharmacovigilance and ADR reporting. Approximately 6–10% of ADRs are reported, according to estimates.6 Therefore, underreporting has been a significant barrier to ADRs being reported voluntarily, which presents a significant challenge for pharmacovigilance efforts and has a detrimental effect on public health. Since an effective system of ADR reporting is crucial to improve patient care and safety, as well as overall health, healthcare practitioners are expected to view ADR reporting as a professional obligation.6 Most patients' first points of contact with healthcare are community pharmacists since they are the most accessible. Community pharmacists are readily available sources of medical treatment. They are reputable, well-educated healthcare experts,7 who provides drug related services for the purpose of promoting effective and safe drug therapy in order to improve patient health.8
Many factors are associated with ADR reporting among health professionals. These factors have been broadly classified as personal and professional characteristics of the healthcare provider and their knowledge and attitudes to reporting.9 Inman has summarised these factors as the ‘seven deadly sins.’10 His description of the ‘sins’ include: attitudes relating to professional activities (financial incentives: rewards for reporting; legal aspects: fear of litigation or enquiry into prescribing costs; and ambition to compile or publish a personal case series) and problems associated with ADR-related knowledge and attitudes (complacency: the belief that very serious ADRs are well documented by the time a drug is marketed; diffidence: the belief that reporting an ADR would only be done if there was certainty that it was related to the use of a particular drug; indifference: the belief that the single case an individual provider might observe could not contribute to medical knowledge; and ignorance: the belief that it is only necessary to report serious or unexpected ADRs), and excuses made by professionals (lethargy: the procrastination and disinterest in reporting or lack of time to find a report card and other excuses).10
Objectives of study
- To establish the level of knowledge of pharmacists with regards to ADRs and ADR reporting.
- To document pharmacists’ perception of ADR reporting and pharmacovigilance.
- To evaluate ADR reporting by community pharmacists in Lagos State.
- To identify the factors that influence ADR reporting by community pharmacists in Lagos state.
- To solicit from pharmacists their views on how ADR reporting can be improved.
Justification
Despite the advantages of using medications, there are also adverse effects that comes with their use, that has led to challenges in tracking Adverse Drug Reactions (ADRs).11 Community Pharmacists, who are the most commonly consulted healthcare provider, are in a good position to record ADRs as part of their regular practice.3 Studies have shown that a significant amount of ADRs are not being reported by healthcare personnel due to various reasons, spontaneous reporting of adverse drug reactions is extremely important to healthcare systems, underreporting is therefore a major obstacle to ADR reporting,6 hence it is important to recognize various factors affecting adverse drug reaction reporting, so as to be able to device means to tackle them.
Method
Research design
A cross-sectional survey design involving community pharmacists in Lagos state was carried out.
Study sample population
For this study, a minimum of 30 community pharmacists in each of the areas under study were randomly selected (120 respondents). Of the 20 Local Government Areas in Lagos State, Nigeria, four areas were selected for the survey (Ikeja, Surulere, Victoria Island and Ikorodu) to represent the various population density outlooks of the state. Community pharmacists in these areas were visited and requested to consent to the study.
Study instrument
For the purpose of the study, a survey questionnaire was used. This questionnaire was developed to collect appropriate data consisting of 40 open and closed questions. The questions were drafted to explore the knowledge, attitude and practice of ADR reporting, and the factors influencing ADR reporting by the respondents. The questionnaire was validated by the supervising lecturer and one other expert in the ADR field. The questionnaire was structured based on the objectives of the study in such a way that each section had a minimum of 5 questions to obtain reliable information on the particular objective.
Data analysis
After collection of the questionnaires, data was entered unto Microsoft Excel and analysed using SPSS 15. Frequency and percentage were used in analysing the socio demographic data of the respondents, the knowledge of the respondents on ADR and its reporting, the perception and in evaluation of the respondents’ knowledge of ADR reporting.
Results
Out of the 120 questionnaires given out only 118 was completely filled and analyzed.
Table 1: Demographic Characteristics of the Respondents
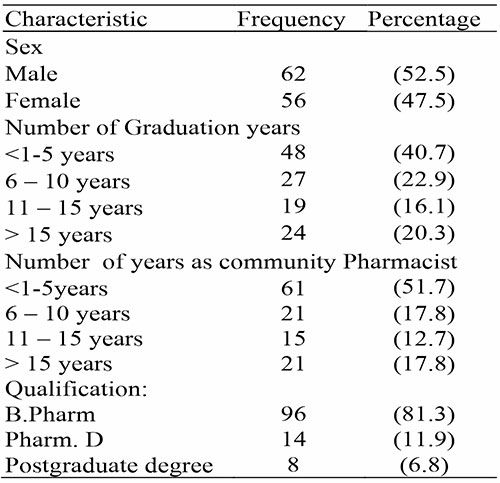
Table 2: Perception of ADR Reporting
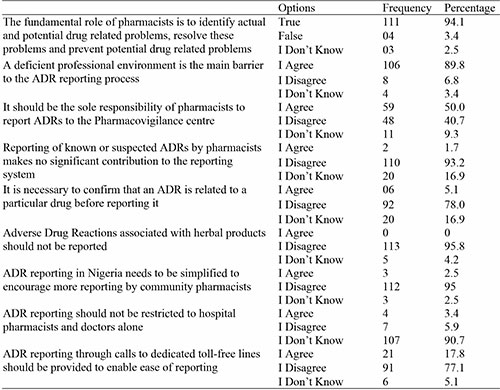
Table 3: Pharmacists’ Practice on ADR Reporting
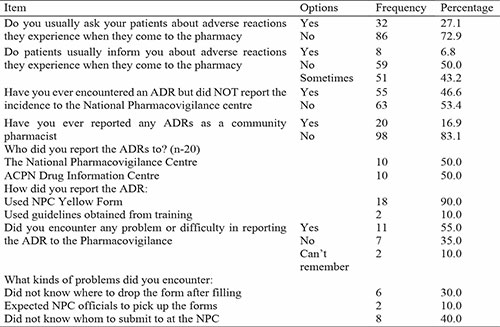
Table 4: Barriers to ADR Reporting
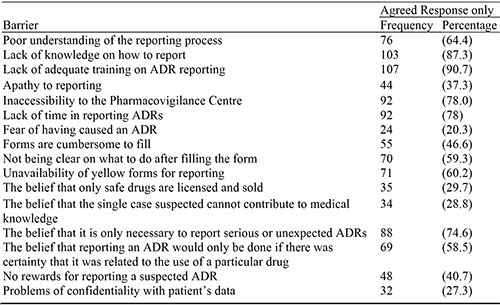
Table 5: How to Improve ADR Reporting
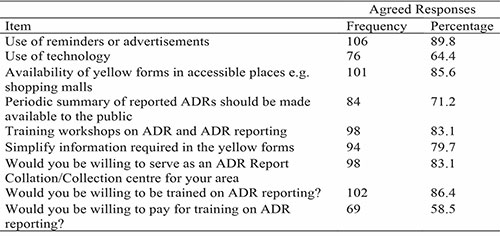
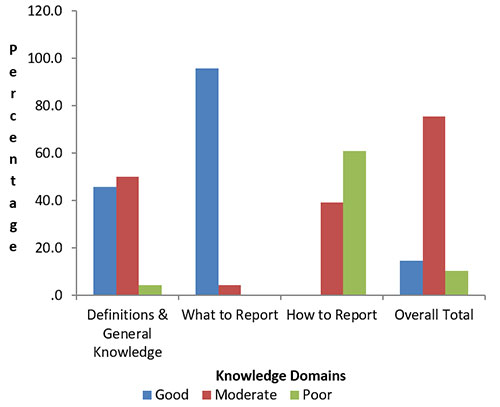
Figure 1: Knowledge of ADR Reporting by Community Pharmacists (Percent of correct responses)
Discussion
ADRs are primarily prevented globally through spontaneous reporting, whose limiting aspect is underreporting. ADR underreporting is correlated with health workers' knowledge, attitudes, and reporting practice.12 In this research most of the community pharmacists could define what an ADR is, and many agreed that the most common way that regulatory bodies collect ADR information for medicines once they are in the market is through voluntary, spontaneous reporting structures. However, knowledge is very limited in the area of how to report ADR, this agrees with the study done in Australia by Raymond et al. (2018).
In the area of pharmacists’ perception of ADR reporting, this research shows that pharmacists have good perception about ADR and its reporting, this agrees with the study done by Hadi et al. (2017),13 as well as the study done by Joubert & Naidoo. (2016),14 but does not agree with the study done by Raymond et al. (2018). In general, pharmacists indicated a readiness to report ADRs and thought that doing so was part of their duty as practicing professionals. However, a number of obstacles to reporting ADRs that pharmacists encounter have also been noted.13 Pharmacovigilance was viewed favorably by the majority of responders. This response is consistent with past studies that discovered pharmacists are quite optimistic about the possibility of working with pharmacovigilance situations.14 Majority of the respondents in this research regarded pharmacovigilance as a fundamental role of pharmacists, and that deficient professional environment is the main barrier to ADR reporting. Half of the respondents agreed that it should be the sole responsibility of the pharmacists to report ADRs to pharmacovigilance centers, while most agreed that ADR associated with herbal products should also reported.
In the area of practice, we found that only 16.9% have ever reported ADRs as a community pharmacist, which shows a low reporting rate of ADRs this agrees with the study done by Raymond, et al. (2018), this is not consistent with the study done by Hughes & Weiss, (2019),15 which shows 52% reporting rate.
Studies from Europe, Asia, and Africa showed reporting rates of between 14 and 44% to their respective national ADR reporting centres, which are broadly consistent with the low ADR reporting rates. The various working conditions and difficulties community pharmacists encounter around the world may be the cause of this significant variation in ADR reporting rates.3 Most of the participants reported through the use of the yellow form.
Majority of the pharmacists in this research agreed to engage their patients to know the adverse reaction they experience when they come to the pharmacy. This indicates that pharmacists are knowledgeable and interested in ADR reporting but there exists barriers affecting reports of ADR.
As to barriers affecting ADR reporting, the results of this study shows that lack of adequate training on ADR reporting is the greatest barrier as most respondents agreed to this, this can be compared to the study of Cheema et al., 2017,16 Which had Majority of the pharmacists identified training and information about what to report as one of the facilitators of pharmacovigilance reporting. Another barrier is lack of knowledge on how to report, this agrees with the study by Raymond et al., 2018, which attributes low rate of ADR reporting to limited knowledge of pharmacovigilance, also more than half of the respondents agreed that professional bodies should organize workshop and training workshops on ADR reporting. It is clear that pharmacists did not obtain sufficient education as part of their continuous professional development because knowledge ratings were not higher in responders with post-graduate degrees or those with more years of experience. This is a crucial unmet requirement.3 Other major barriers includes, lack of time in reporting ADRs, this can be compared with the study carried out by Joubert and Naidoo, (2016),14 in which half of respondents found reporting to be time consuming, whilst over 38% and 35% indicated that they do not know how to report or where to report respectively, as well as poor understanding of the reporting process this is as well consistent with the study carried out by Joubert and Naidoo,14 (2016). Some of the barriers cited in this study are consistent with barriers found in similar studies, these include how to report, where to report, where to obtain a form, the reporting form not user friendly and poor feedback.14
Almost all the respondents showed interest in education and training. Most of the respondents also have the willingness to serve as an ADR Report Collation/Collection center for their area. Continuous Professional Education, training and refresher courses were the most cited means of improving ADR reporting. This certainly shows that the pharmacists are willing to improve their knowledge of ADR reporting and increase their participation in the ADR reporting scheme and the other available means of reporting. Other methods were recommended by the respondents such as instituting and encouraging feedback between patients, prescribers and dispensers of drugs, receiving reminders and increased awareness from ADR Monitoring Committee, and increasing the awareness of other healthcare professionals on reporting.
Conclusion
This research clearly shows that there is a good knowledge of the concepts of ADR, Pharmacovigilance and the reporting of ADRs by community pharmacists in Lagos state. There is however a gap between the knowledge and the actual practice of ADR reporting as many were not reporting.
This study was able to evaluate ADR reporting by community pharmacists in Lagos state and has shown that many of them do not pay attention to reporting ADRs due to various constraints like being too busy attending to patients and the belief that only safe drugs are licensed and sold.
References
- Pagel WP. An introduction to philosophical medicine in the era of the renaissance. Swedish Pharm J. Karger-basel, Switzerland. 1998; 4(37):67-68.
- Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med (Lond). 2016 Oct;16(5):481-485. doi: 10.7861/clinmedicine.16-5-481. PMID: 27697815; PMCID: PMC6297296.
- Li R, Curtain C, Bereznicki L, Zaidi STR. Community pharmacists' knowledge and perspectives of reporting adverse drug reactions in Australia: a cross-sectional survey. Int J Clin Pharm. 2018 Aug;40(4):878-889. doi: 10.1007/s11096-018-0700-2. Epub 2018 Aug 10. PMID: 30097819; PMCID: PMC6132965.
- World Health Organization (WHO). The importance of Pharmacovigilance - Safety Monitoring of Medicinal Products; chapter 5. Pharmacovigilance in clinical practice. Available at http://apps.who.int/medicinedocs/en/d/Js4893/6.html. Accessed: 23-01-2013.
- Jefferys D, Leakey D & Lewis J. Adverse effects of drugs in use. British Medical Journal 2006;12(45):151-6
- Adisa R, Omitogun TI. Awareness, knowledge, attitude and practice of adverse drug reaction reporting among health workers and patients in selected primary healthcare centres in Ibadan, southwestern Nigeria. BMC Health Serv Res. 2019 Dec 3;19(1):926. doi: 10.1186/s12913-019-4775-9. PMID: 31796034; PMCID: PMC6889459.
- Rasaq KO, Afeez BO. Differentiated service delivery and the community Pharmacists' roles in achieving UNAIDS 2030 target in Nigeria. Saudi Pharm J. 2021 Aug;29(8):815-819. doi: 10.1016/j.jsps.2021.06.003. Epub 2021 Jun 15. PMID: 34408543; PMCID: PMC8360771.
- Moullin JC, Sabater-Hernández D, Fernandez-Llimos F, Benrimoj SI. Defining professional pharmacy services in community pharmacy. Res Social Adm Pharm. 2013 Nov-Dec;9(6):989-95. doi: 10.1016/j.sapharm.2013.02.005. Epub 2013 Apr 13. PMID: 23591411.
- Lexchin J. Is there still a role for spontaneous reporting of adverse drug reactions?.CMAJ Canadian Medical Association journal 2006;174(2), 191–192. https://doi.org/10.1503/cmaj.050971
- Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009; 32(1):19-31. doi: 10.2165/00002018-200932010-00002. PMID: 19132802.
- Vivekanandan K, Pranay K, Prabhakar M, Gyanendra NS. System of adverse drug reaction reporting: What, where, how, and whom to report?.indian J crit care med. 2015 Sep; 19(9): 564- 566
- Adedeji WA, Adegoke AB, Fehintola FA. Adverse drug reactions reporting practice and associated factors among community health extension workers in public health facilities, Southwest, Nigeria. Pan Afr Med J. 2021 Nov 17; 40:165. doi: 10.11604/pamj.2021.40.165.28574. PMID: 34970407; PMCID: PMC8683451.
- Hadi MA, Neoh CF, Zin RM, Elrggal ME, Cheema E. Pharmacovigilance: pharmacists' perspective on spontaneous adverse drug reaction reporting. Integr Pharm Res Pract. 2017 Mar 22; 6:91-98. doi: 10.2147/IPRP.S105881. PMID: 29354555; PMCI D: PMC5774327.
- Joubert, MC, Naidoo P. Knowledge, perceptions and practices of pharmacovigilance amongst community and hospital pharmacists in a selected district of North West Province, South Africa. Health SA Gesondheid.2016; 21. 238-244.10.1016/j.hsag.2016.04.005.
- Hughes ML, Weiss M. Adverse drug reaction reporting by community pharmacists-The barriers and facilitators. Pharmacoepidemiol Drug Saf. 2019 Dec; 28(12):1552-1559. doi: 10.1002/pds.4800. Epub 2019 May 27. PMID: 31131966.
- Cheema E, Haseeb A, Khan TM, Sutcliffe P, Singer DR. Barriers to reporting of adverse drugs reactions: a cross sectional study among community pharmacists in United Kingdom. Pharm Pract (Granada). 2017 Jul-Sep; 15(3):931. doi: 10.18549/PharmPract.2017.03.931. Epub 2017 Aug 8. PMID: 28943977; PMCID: PMC5597805.