Efficacy of oral Tranexamic Acid in reducing blood loss during primary total hip arthroplasty: A comparative study
Essien UE1, Inyang UC1, Itakpe SE2, Lawal WO2, Dim EM1, Usendiah IB1
Abstract
Background: Perioperative blood loss is a major concern for patients undergoing total hip arthroplasty. Intravenous Tranexamic acid is commonly used to reduce blood loss and need for blood transfusion following total hip replacement. Recent studies have shown the efficacy of oral tranexamic acid in reducing perioperative blood loss in total hip replacement.
Method: A total of 69 patients undergoing primary total hip replacement in National Orthopaedic Hospital, Igbobi, Lagos, were recruited. Patients were randomized to receive 2gram oral tranexamic acid 2hours before incision or 1-gram intravenous tranexamic acid 10minutes before incision. Primary outcome measure was reduction in haemoglobin concentration.
Results: There was no significant difference in mean reduction in haemoglobin concentration in the oral tranexamic acid group compared to intravenous tranexamic acid group (3.78g/dl ±1.27 and 3.61g/dl ±0.54 respectively, P- 0.49). There was no significant difference between total blood loss and requirement for blood transfusion in the two groups.
Conclusion: Oral and intravenous routes of tranexamic acid administration have comparable efficacy in reducing blood loss and requirement for blood transfusion. Oral tranexamic acid is cheaper, easier to administer and should be considered as a safe and effective alternative to intravenous tranexamic acid.
Keywords: Oral tranexamic acid, Intravenous tranexamic acid, Total hip arthroplasty, Perioperative blood loss.
Introduction
Perioperative blood loss during total hip arthroplasty is a major concern for both the surgeon and the patient. Total hip arthroplasty is associated with substantial perioperative blood loss usually greater than 1000mls, resulting in post operative anemia and increase requirement for blood transfusions.1-3 Ugbeye (2017) revealed a mean intraoperative blood loss of 1200mls following primary total hip arthroplasty, with 92.2% of patients requiring intraoperative blood transfusion.2
Tranexamic acid is regarded as one of the most important drugs used in reducing blood loss in total hip arthroplasty,4 several studies have shown it to be effective in reducing perioperative blood loss and transfusion requirement in both primary and revision hip arthroplasty.5,6 It competitively and reversibly complexes with plasminogen, blocking its lysine binding sites thereby inhibiting the conversion of plasminogen to plasmin thus promoting clot stabilization.7 This prevents the degradation of fibrin clot by plasmin resulting in reduction in blood loss.
The purpose of this study was to determine the efficacy of oral tranexamic acid in reducing blood loss following primary total hip arthroplasty when compared with intravenous tranexamic acid.
Materials and methods
This double blinded prospective study was conducted at the National Orthopaedic Hospital Igbobi, Lagos, from April 2018 to March 2019. All patients for primary total hip arthroplasty who consented to the study within the study period were recruited. Patients undergoing revision hip arthroplasty surgery, allergic to tranexamic acid, with past medical history of thromboembolic event, renal impairment or bleeding disorders were excluded from the study.
Ethical consideration
Approval for the study was obtained from the Ethics and Research Committee of the National Orthopaedic Hospital Igbobi,
Sampling technique
The sample size was calculated using Taro Yamane formula for estimation of sample size8
n = N / [1 + N (e)2]
Where,
n = Samples size, N = Total population (total number of patients who had primary total hip replacement in National Orthopaedic Hospital Igbobi, in the previous year (2016), documented as 61 in the theater register), e = Error tolerance, margin of error of 0.05 was used with 95% confidence interval. The calculated sample size was 53.
A total number of sixty-nine patients were recruited into the study. They were randomized into groups A and B, using simple random sampling. Seventy pieces equal sized cards were labeled A or B and placed in an opaque bag, 35 for each study group. Patients who had given consent to participate in the study, were asked to pick a card from the bag. Once drawn, the card picked was not returned to the bag and the patient was assigned to a group based on the inscription on the card drawn. Patients who picked cards labeled A received oral tranexamic acid while those who picked cards labeled B received intravenous tranexamic acid. Patient’s hospital numbers were used for identification.
Procedure
For this study the dose of intravenous tranexamic acid used was 15mg/kg approximated to 1gram bolus dose. Single dose of intravenous tranexamic acid was given 10mins before the skin incision. The dose for oral tranexamic acid used was 2grams equivalent to four tablets (each tablet contains 500mg) administered two hours prior to skin incision.
Tablet vitamin C was given to patients receiving intravenous tranexamic acid and normal saline was administered intravenously to patients receiving oral tranexamic acid. These were used as placebo to ensure patients in both groups received medications in the ward and in the operating room. These medications are safe and are not known to influence haemostasis. Tablet vitamin C has identical shape and colour as oral tranexamic acid, while normal saline and intravenous tranexamic acid are both odorless and colourless liquids.
Patients in Group A received four tablets of 500mg tranexamic acid, equivalent to 2grams of oral tranexamic acid in the ward, administered by the ward nurse two hours before surgery with a sip of water. In the operating room 10mls of normal saline (placebo) was administered intravenously 10mins before skin incision by the anesthetist.
Patients in Group B received four tablets of vitamin C (placebo) in the ward, administered by the ward nurse two hours prior to surgery with a sip of water. In the operating room intravenous tranexamic acid 1gram (10mls) was administered 10mins before skin incision by the anesthetist.
All patients received prophylactic antibiotics, spinal anaesthesia and active suction drain at closure of wound. Surgery was performed using either the posterior or lateral approach to the hip.
Intraoperative blood loss was calculated by estimating the volume of blood in the gauze packs and swabs used, and the volume of blood in the suction reservoir. Blood in the gauze packs and swabs were estimated based on preliminary estimation of blood volume in a partially and fully soaked abdominal pack and swab and determining the difference in wet and dry weight (Table 1). Blood on the drapes and on the floor were also estimated and included in the total blood loss.
Outcome measures
Primary Outcome Measures:
- Reduction of hemoglobin concentration, defined as the preoperative hemoglobin minus lowest of the postoperative hemoglobin measured at 12hours, 24hours and 48hours.
Secondary Outcome Measures:
- Number of blood units transfused: total units of blood transfusions intraoperatively and within 72hrs of the postoperative period.
- Intraoperative blood loss: estimated from the total number of abdominal packs and swabs used and the total quantity of blood in the suction reservoir at the end of surgery.
- Total drain output - the volume of blood in a calibrated reservoir connected to a drain placed in the hip, which was measured and recorded at 48hours.
- Cost variation between intravenous and oral tranexamic acid.
Data analysis
Data was analyzed using the IBM Statistical Package for Social Sciences (SPSS) version 20 for Windows. Following analysis, t-test was performed to determine any statistically significant differences in outcome between the two methods of administration of tranexamic acid.
Results
Sixty-nine patients were recruited for this study. Sixty-three patients completed the study, 32 patients in the oral tranexamic acid group and 31 patients in the intravenous tranexamic acid group.
There was no significant difference in the demographic data among the respondents in both groups. Respondents were between the ages of 30 and 75years, with females accounting for 63.5%.
Table 1: Volume of blood loss into abdominal packs and gauze

Patients received standard postoperative care for total hip replacement. Blood samples were collected for hemoglobin check at 12hours, 24hours and 48hours post-surgery. Total output from wound drain was measured and recorded at 48hrs. Total units of blood transfused within 72hrs post-surgery was calculated and recorded. Blood transfusion was based on 2016 guideline of the American Association of Blood Bank (AABB).9J
Table 2: Socio-demographic Characteristics of Respondents
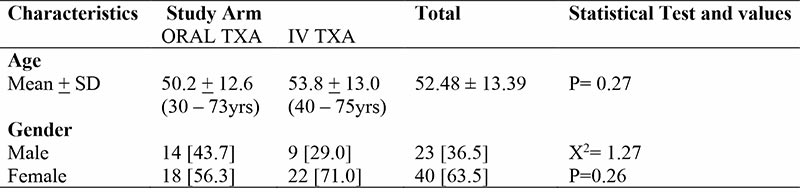
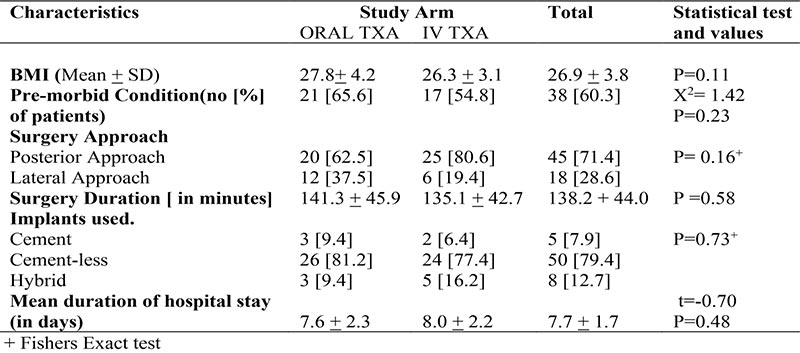
There was no significant difference in the clinical characteristics of respondents. Mean BMI was 26.9 ± 3.8 with range from 17.5 – 35.2. About 60% of respondents had co- morbidities. In 71.4% of respondents posterior approach was used while 28.6% had direct lateral approach. Average duration of surgery was 138.2mins ± 28.6. About 79% of respondents had cementless total hip prosthesis and mean duration of hospital stay among the two groups was 7.7days ±1.7.
Table 3: Clinical Characteristics of Respondents
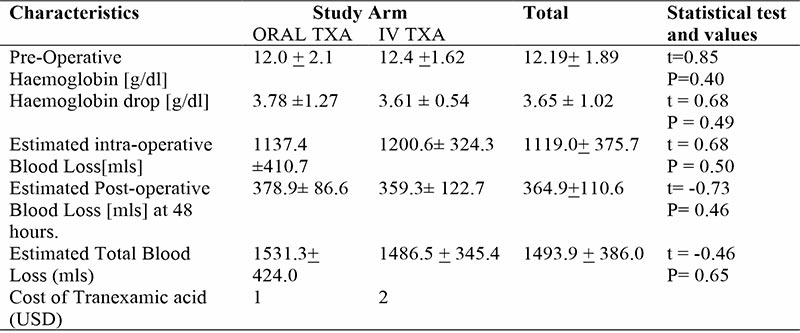
There was no significant difference in the outcome measures. The mean pre-operative haemoglobin was 12.19g/dl ± 1.89, while mean haemoglobin concentration drop was 3.65g/dl ±1.02. The mean intra-operative blood loss was 1119.0mls ±375.7. The mean estimated post-operative blood loss at 48hours was 364.9mls ±110, while mean total blood loss was 1493.9mls ±386.0.
Table 4: Outcome Measures
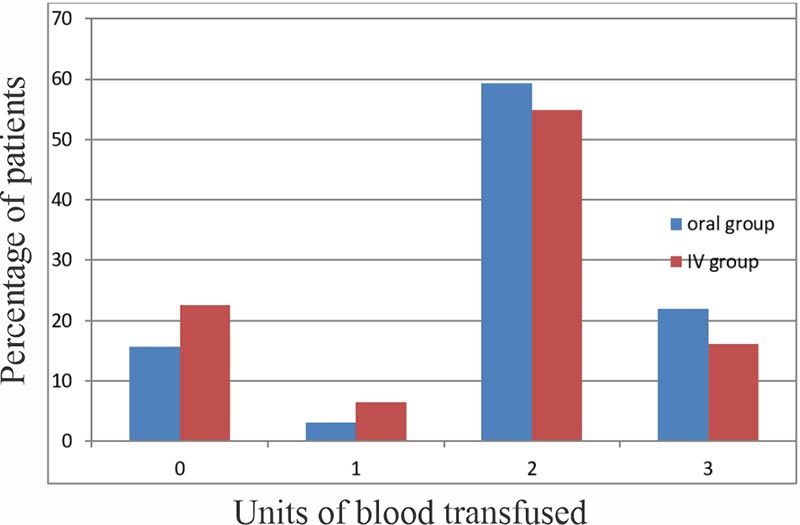
There was no significant difference in rate of blood transfusion between the two groups, P value 0.34. In the oral group, 5(15.62%), 1(3.13%), 19(59.38%) while 7(21.87%) of respondents were transfused 0, 1, 2, and 3 units of blood respectively. In the intravenous group, 7(22.58%), 2(6.48%), 17(54.84%) and 5(16.13%) of respondents were transfused 0, 1, 2 and 3 units of blood respectively.
The cost of 2grams of oral tranexamic acid is about half the cost of 1gram of intravenous tranexamic acid.
Figure 1: Chart showing percentage of patients and unit of blood transfused
Discussion
Tranexamic acid is widely used in total hip arthroplasty surgeries and is known to be effective in decreasing perioperative blood loss and requirement for blood transfusion. The usefulness of tranexamic acid in total hip replacement is well established. However, there is no consensus on the optimal dosage, frequency of administration and route of administration. Intravenous route is the most used route of administration.
Sixty-nine patients were recruited for this study, 6 patients were disqualified for not receiving medications in the ward. Sixty-three patients completed this study. There was no significant difference in the demographic data between the two groups. The mean age of patients recruited was 52yrs ±13.39, with age range from 30 –75yrs. This is similar to findings reported in a randomized controlled trial by Kayupov et al who reported a mean age of 57yrs ± 11.1 A total of 63.5% of respondent were females while men were 36.5%, giving a male to female ratio of 1:1.7, similar to findings reported by Ugbeye et al in his study of 41 patients, females accounted for 63.4%, with a male to female ratio of 1:1.7.2
The primary outcome measure was haemoglobin concentration drop between the oral and intravenous tranexamic acid group. Haemoglobin drop reflects the total blood loss and is also a trigger for blood transfusion. The mean haemoglobin drop was 3.78g/dl for the oral tranexamic acid group and 3.61g/dl for the intravenous tranexamic acid group. This is similar to findings by Kayupov et al who reported a drop of 3.67g/dl and 3.53g/dl for the oral and intravenous tranexamic groups respectively, in a randomized controlled trial of 83 patients.1 Luo et al reported similar results in his randomized controlled trial of 180 patients, with haemoglobin drop of 3.48g/dl and 3.58g/dl for the oral and intravenous tranexamic groups respectively.10 Luo et al in his study however, measured the post operative haemoglobin at 24hours and 72hours post-surgery unlike this study. However, a study by Nagra et al reported that there was no significant difference in haemoglobin drop between D2 and D3 post operative days.11 This study showed there was no significant difference in the haemoglobin drop in the two groups with P value 0.62. This was similar to findings reported by Kayupov et al and Luo et al.1,10
There was no significant difference in total blood loss between the oral tranexamic acid and intravenous tranexamic acid group, 1531.5mls and 1486.3mls respectively. Ugbeye et al in the evaluation of intra- and post-operative blood loss in total hip arthroplasty reported mean total blood loss of 1786.2mls which was higher than findings in this study.2 This result is in keeping with the documented efficacy of tranexamic acid in reducing blood loss following total hip arthroplasty.3,12 Kayupov et al in his study reported findings similar to this study, with 1339mls and 1301mls for oral and intravenous tranexamic acid groups respectively.1 The study by Luo et al reported less blood loss in the oral and intravenous tranexamic acid groups, 1004mls and 1032mls respectively.10 However, there was no significant difference between the two groups. This shows that single dose of 2grams oral tranexamic acid and 1g intravenous tranexamic acids were equally effective in reducing total blood loss. Husted et al in his study reported a mean total blood loss of 814ml, which was lower than blood loss recorded in this study.13 Husted in his study used 10mg/kg of tranexamic acid given as bolus intravenous injection followed by continuous infusion of 1mg/kg/hr for 10hours.13 This may have contributed to much more reduction in blood loss in his study compared to the intravenous group in this study. This seems to suggest multiple doses may result in more reduction in total blood loss compared to single dose of intravenous tranexamic acid. This will benefit from further research.
There was no significant difference in intraoperative blood loss between the two groups, 1137.4mls and 1200.6mls for the oral tranexamic acid and intravenous tranexamic acid groups respectively. Ugbeye et al reported a mean post-operative blood loss of 574.3mls in his study, evaluation of blood loss in total hip replacement.2 In this study, the mean post-operative blood losses between the oral tranexamic acid and intravenous tranexamic acid groups were 378.9mls and 359.3mls respectively. This was lower than findings recorded by Ugbeye et al, indicating that the use of tranexamic acid was effective in reducing both intra-operative and post-operative blood loss. This study also showed there was no significant difference in mean post-operative blood loss between the two groups, P value 0.46. Luo et al in his study did not use post operative suction drain, hence post operative blood loss could not be estimated.10 Similar study done by Kayupov et al did not consider post operative blood loss as an independent factor; this made it difficult to compare this finding.1
Trigger for blood transfusion was symptomatic haemoglobin drop based on American association of Blood Bank (AABB) guidelines 2016.9 In the oral tranexamic acid group, 5(15.62%), 1(3.13%), 19 (59.38%) while 7(21.87%) of respondents were transfused 0, 1, 2, and 3 units of blood respectively. In the intravenous tranexamic acid group, 7(22.58%), 2(6.48%), 17(54.84%) and 5(16.13%) of respondents were transfused 0, 1, 2 and 3 units of blood respectively. There was no significant difference between the two groups in respect to the requirement for blood transfusion, P value 0.35. Luo et al reported there was no significant difference in the two groups; however, majority of patients in his study did not receive blood transfusion.10 One respondent received a unit of blood in the post operative period, all other transfusion 98.41% was in the intra-operative period. This is similar to findings by Luo et al who reported that all the transfusion was done intra-operatively.10
The cost of the dose of intravenous tranexamic acid used in this study was double the price of the dose of oral tranexamic acid, 2 USD and 1 USD respectively. This is similar to findings by Kayupov et al and Luo et al.1,10 This appears to be the main advantage of oral tranexamic acid over intravenous tranexamic acid.
Conclusion
This study suggests that a single dose of 2grams oral tranexamic acid is comparable to bolus dose of 1gram of intravenous tranexamic acid in reducing haemoglobin drop, perioperative blood loss and requirement for blood transfusion in patients undergoing primary total hip arthroplasty. Considering the efficacy, safety, ease of administration and low cost of oral tranexamic acid, oral tranexamic acid is recommended as a safe and effective alternative to intravenous tranexamic acid in reducing haemoglobin drop, perioperative blood loss and requirement for transfusion in patients undergoing total hip arthroplasty.
References
- Kayupov E, Fillingham Y A, Okroj K, Plummer D R, Moric M, Gerlinger T L et al. Oral and intravenous tranexamic acid are equivalent at reducing blood loss following total hip arthroplasty. J Bone Joint Surg Am. 2017; 99(5):373-378.
- Ugbeye M E, Lawal W O, Ayodabo O J, Adadavoh I P, Akpan I J, Nwose U. An evaluation of intra- and post-operative blood loss in total hip arthroplasty at the National orthopaedic Hospital, Lagos. Niger J Surg 2017;23;42-46.
- Gandhi R, Evans H M, Mahomed S R, Mahomed N N. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty; a meta-analysis. BMC Research Notes 2013; 6:184.
- Zhang L, Ma J, kuang M, Zhao J, Wang Y, Lu B et al. Comparison of oral versus Intravenous application of tranexamic acid in total knee and hip arthroplasty; A systematic review and meta-analysis. International Journal of surgery 2017;45;77-84.
- Niskanen R O, Korkala O L. Tranexamic acid reduces blood loss in cemented hip arthroplasty: a randomized, double – blind study of 39 patients with osteoarthritis. Acta Orthop. 2005 Dec; 76 (6): 829-832.
- Sukeik M, Alshryda S, Haddad F S, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011 Jan: 93(1): 39-46.
- Okamoto S, Hijikata-Okunomiya A, Wanaka K et al. Enzyme-controlling medicines: Introduction. Semin THromb Hemost. 1997; 23(6): 493-501.
- Singh AS, Masuku MB. Sampling techniques and determination of sample size in applied statistics research: An Overview. International Journal of Economics, Commerce and Management 2014 Nov;2(11):122.
- Carson J, Guyatt G, Heddle N et al. Clinical Practice Guidelines from the AABB - Red Blood transfusion Thresholds and storage. JAMA.2016; 316(16):2025-2035.
- Luo ZY, Wang HY, Wang D et al. Oral vs intravenous vs tropical tranexamic acid in primary hip arthroplasty: a prospective, randomized, double- blind, controlled study. J Arthroplast.2018; 33(3):786 – 93.
- Nagra NS, Holt EM, Popta D, Whiteside S. An analysis of post operative haemoglobin levels in patients with fractured neck of femur. Traumatologica Turcica (50)5:(10)2016, 507 – 513.
- Claeys M A, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg.2007 Jul-Aug; 107(4):397-401.
- Husted H, Blond L, Sonne-Holm S et al. Tranexamic acid reduces blood loss and transfusions in primary total hip arthroplasty. A prospective randomized double-blind study in 40 patients. Acta Orthop Scad. 2003; 74: 665-669.