A retrospective histomorphologic study of meningeal masses in Benin City, Nigeria
Udoh MO
Abstract
Background: The rarity of many histologic entities and the common discordance between histologic appearance and biologic behaviour make the diagnosis of meningeal masses challenging. Except for a few common entities like meningioma and meningeal metastases, data on the prevalence of meningeal mass lesions is limited, and most exist as case reports and small case series. This study presents the pattern of meningeal masses in our environment, in the hope of adding to knowledge and awareness of the differential diagnoses of meningeal masses in our environment.
Materials and methods: A retrospective study of meningeal masses histologically diagnosed at the the University of Benin Teaching Hospital, Benin city, Nigeria, based on clinical, demographic and histomorphologic data obtained over a 15-year period.
Results: Total of 140 meningeal masses; 137 neoplastic and 3 non-neoplastic mass lesions. Ages ranged from 3-86 years. Mean age was 45.85 ± 18.16 years. There were 52 males and 88 females with an overall male-female ratio of 1:1.7; but non-meningothelial tumours were more common amongst males. Most common tumour was Meningioma (78.6%), then metastatic tumours (15%), non-meningothelial mesenchymal tumors (4.3%), and hypertrophic pachymeningitis (2.1%). Meningioma, hemangioblastoma and embryonal rhabdomyosarcoma were the most common tumours in children.
Conclusion: Rare histologic entities arising in the meninges, can easily be overlooked in the differential diagnosis of meningeal mass lesions, while the common ones like meningioma and meningeal metastases, are at risk of becoming ‘waste basket’ or ‘safety net’ diagnoses for meningeal tumours. There is need for continuous evaluation and audit of histological diagnoses of meningeal tumours to avoid missing the rare entities that may occasionally be encountered.
Keywords: Dura, Meninges, Meningioma, Metastases, Non-meningothelial, Tumours.
Introduction
Tumours of the meninges are a heterogeneous group of intracranial dural/leptomeningeal-based extra-axial lesions with various biological behaviors which may present clinically as masses lesions or as neoplastic meningitis.1-3 They include meningeal neoplasms both benign and malignant that arise from or secondarily involve the meninges of the brain and spinal cord.1,3 Also to be considered are non-neoplastic proliferations or thickenings of the meninges, the causes of which are many.1 Difficulties in differential diagnosis may arise within this group, especially amongst anaplastic tumours because of the wide morphologic spectrum encountered and the rarity of most individual entities.3,4 They can be diagnostically challenging because of the commonly existing discordance between histologic appearance and behaviour.4
About 20%-50% of intracranial tumours present in the meninges.3,5,6 The most common meningeal tumours are meningiomas.7 Meningiomas account for about 36% of intracranial tumours in women and 20% in men and range from WHO grade 1 to grade 3 lesions.6,7 Dural masses that may mimic meningiomas include: primary neoplastic processes, metastases, granulomatous diseases and infection.7 Primary neoplastic tumours ranging from benign non-meningothelial neoplasms to highly malignant sarcomas (corresponding histologically to WHO grade1-4) are seen here.6 They include mesenchymal, lymphoid, histiocytic, and melanocytic tumors, many of which are rare.2,7 Metastatic tumors and tumor-like masses due to infections, inflammatory, or granulomatous diseases e.g tuberculosis are more frequently encountered.2,7,8
Globally the data on the prevalence of meningeal non-meningothelial mass lesions is limited. Most examples in the literature exist as case reports and small case series. A large study of 1000 cases of resected dural masses reported that 2% of cases initially diagnosed as meningiomas were found to be other pathologies.9 The consideration of or a metastatic dural disease or an anaplastic meningioma in contrast to grade 1 meningioma or Hypertrophic Pachymeningitis, has implications for management and prognosis. Early recognition affords a higher chance of preserving neurological function and quality of life; achieving a longer recurrence free interval; or preventing recurrence; through appropriate early treatment.10,17
A recent review of 119 articles from 24 sub-saharan African countries on adult brain tumours, shows significant heterogeneity in the quality of evidence and reporting and highlight the need for rapid and sustainable investment in brain tumor research capacity in sub-saharan Africa.11 This study presents the demographic, clinical and histomorphologic pattern of meningeal tumours in a major referral hospital in Nigeria’s Niger Delta region, in the hope to add to knowledge and awareness of the differential diagnoses of meningeal masses in our local environment, in light of the relative rarity of some of the entities and paucity of data on them.
Materials and methods
A retrospective study of surgical pathology meningeal tumor specimens histologically evaluated and diagnosed at the histopathology department of the University of Benin teaching Hospital, Edo State, Nigeria, over a 15-year period (January 2007 and December 2021) was done.
Records on all biopsies and resections of intracranial space-occupying meningeal lesions excised from neurosurgical patients, were the materials for this study. Clinical and demographic data was obtained from request cards and the surgical day books of the department. All diagnoses were made on 3–5µm haematoxylin and eosin stained histologic sections, prepared from formalin fixed, paraffin embedded blocks of tissue. Tumour classification is based on 2016 WHO Classification of Tumours of the Central Nervous System. Ancillary special histochemical and immunohistochemical stains were employed as needed/available.
Statistical analysis of data was done using the Statistical Programme for Social Sciences, version 20 (SPSS Inc, IL, USA) and observations compared with findings in the literature.
Confidentiality of the identity of the patients and personal health information was maintained.
Results
A total of 140 meningeal tumour specimens -124 cranial and 16 spinal tumours- were submitted for histologic examination during the 15 year period under review. The total number of surgical specimens received in the department within the same period was 43,204, of which 856 were Central nervous system Surgical pathology Specimens. Meningeal tumours therefore represent 0.32% of surgical Pathology specimens received, and 16.4% of CNS specimens evaluated.
Age and Sex
There were 52 males and 88 females with an overall male-female ratio of 1:1.7 The age range of patients was between 3 and 86 years. Mean age for all meningeal lesions was 45.85 ± 18.16 years. Children below 18years made up 7.8% of patients. Age trends are as shown in Figure 1.
Figure 1: Age distribution chart
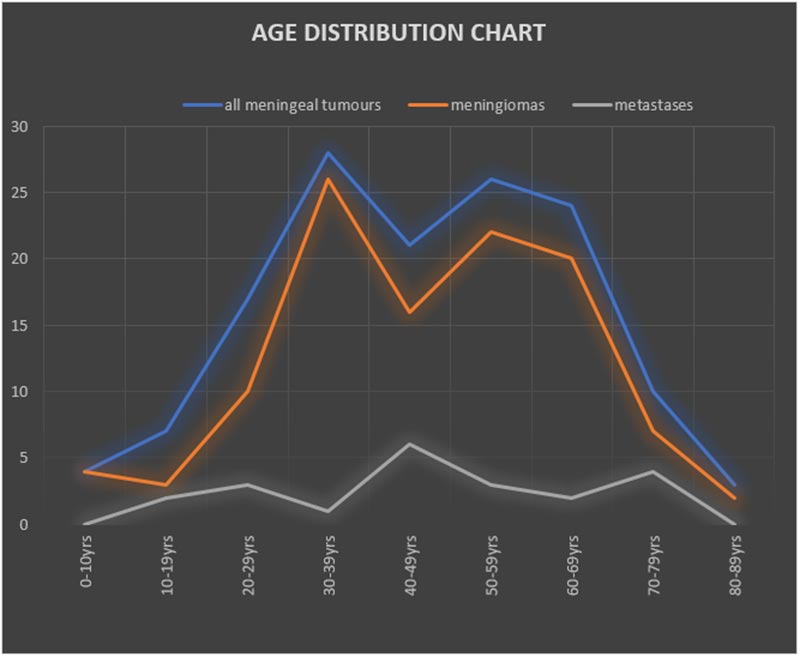
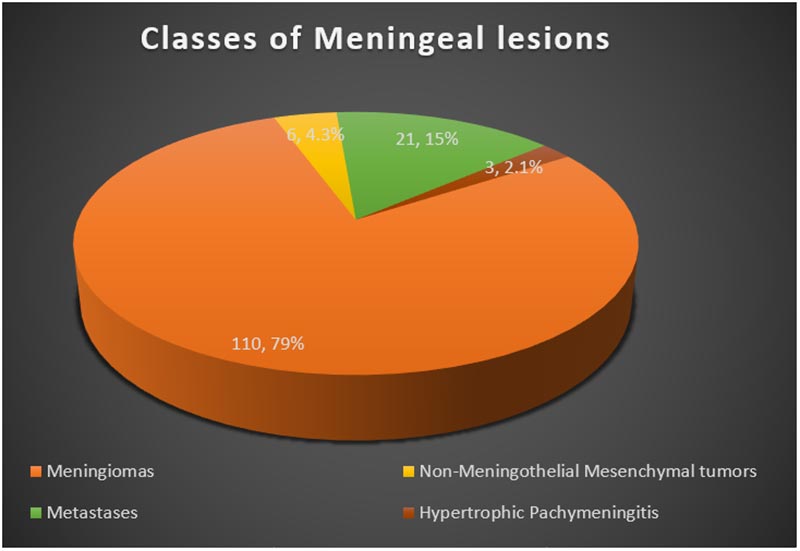
Figure 2: Classes of meningeal lesions
Histological types
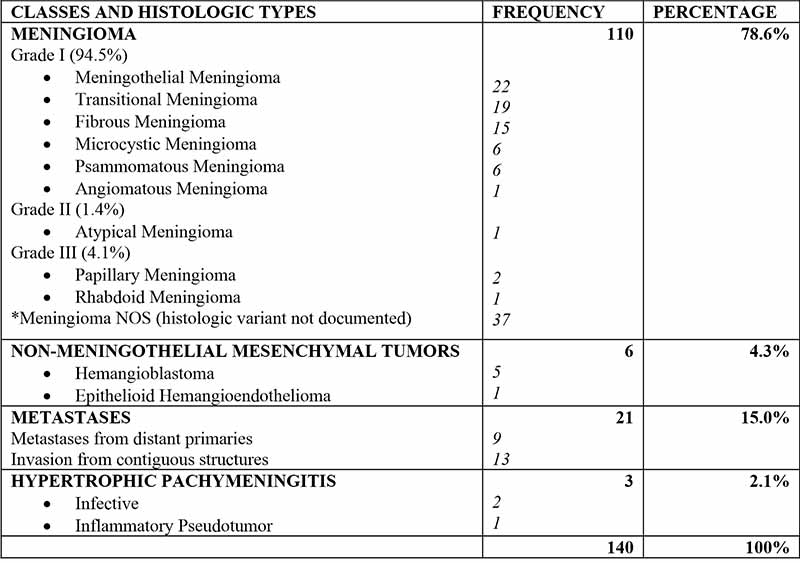
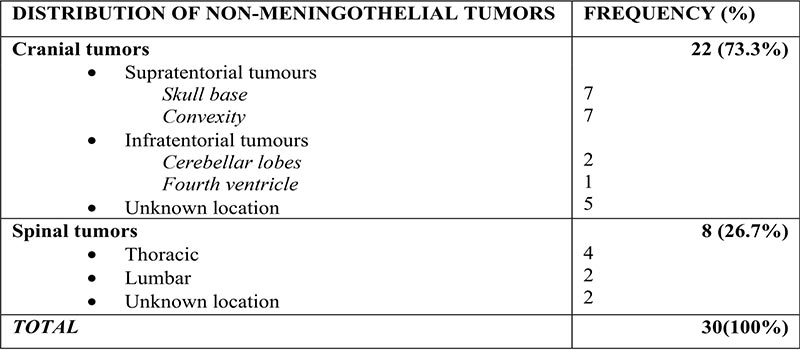
The majority of meningeal tumors evaluated were Meningiomas - 110 cases (78.6%), followed by metastatic tumors - 21 (15%), non-meningothelial mesenchymal lesions - 6 (4.3%), and hypertrophic pachymeningitis-3 (2.1%). The commonest tumours in children were Meningioma, followed by hemangioblastoma and embryonal rhabdomyosarcoma. Types and Histological details are as shown in Figure 1 and Table I.
Table I: Classes and histologic types of Meningeal lesions
Meningiomas
There were 110 Meningiomas constituting 78.6% of meningeal lesion examined and the commonest tumor both in adults and children. There were 33 males and 77 females giving a sex ratio of 1:2.3. The age range of patients with meningioma was 3-86years. The peak age was the 4th decade, with a lesser peak in the 6th decade. Mean age was 46.27 ± 17.57 years. Most meningiomas (94.5%) were grade 1 lesions, with 1.4% and 4.1% being grade 2 and grade 3 lesions respectively. Six meningiomas (5.5%) were seen in children below 18years of age.
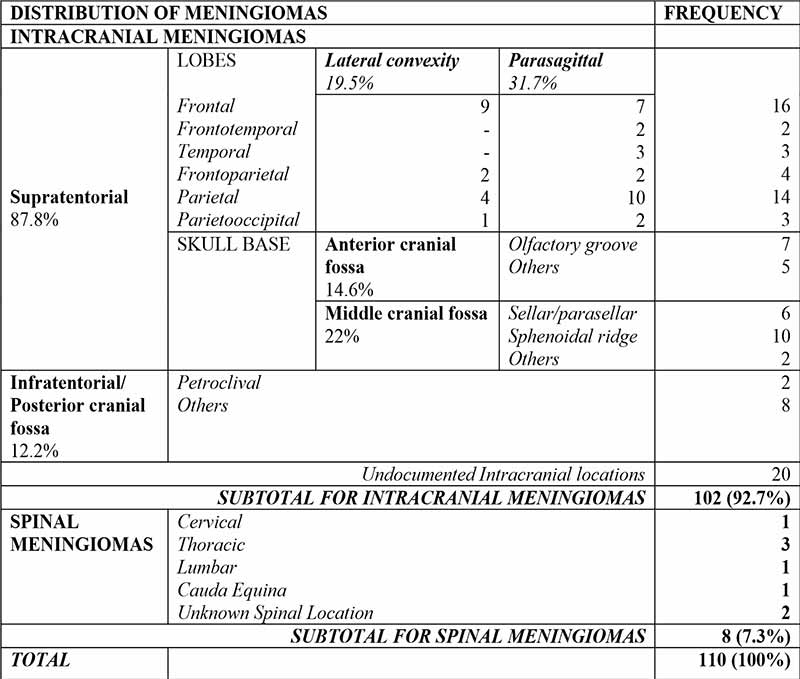
There were 102(92.7%) intracranial and 8(7.3%) spinal meningiomas. Amongst intracranial meningiomas with documented locations, 87.8% were Supratentorial and 12.2% were infratentorial. Common locations include the frontal and parietal lobes, Parasagittal areas, the sphenoidal ridge, and anterior skull base. Specifics are as displayed in Table II.
Laterality was documented in two-thirds of cases, amongst which 40.8% were right sided; 36.6% were left sided; 11.3% were midline, and another 11.3% of cases had bilateral lesions.
Spinal Meningiomas were most common in the Thoracic region (Table II).
Table II: Anatomic distribution of meningiomas
Non-meningothelial lesions
The age range of patients with Non-meningothelial tumors overall was 13-80 years with mean age of 44.28 ± 20.48 years. Male-female ratio was 1.7:1, giving a sex ratio reversal compared to meningiomas. The peak age for non-meningothelial tumors was 20-29 year age group.
Metastatic tumours
Metastatic tumours constitute the majority of Non-meningothelial tumors, thus forming the second commonest class of tumours in this study after meningiomas. They include blood borne Metastases to the meninges as well as invasive tumors arising from contiguous structures secondarily affecting the meninges; the latter group constitutes the majority of meningeal metastases in this study. Sex ratio was 1.3:1 with a male preponderance like other non-meningothelial tumors. The ages of patients ranged between 16 years and 80 years, with a mean age of 48.67 ± 18.70 years. Peak age was the 5th decade with lesser peaks in the 3rd and 8th decades.
Metastatic carcinomas made up 52.4% of meningeal metastases, followed by non-Hodgkin Lymphomas made up 23.8%; other small round blue cell tumours - 14.3%; and others histologic types-9.5%. Majority (62%) of meningeal metastases were from invasive tumours in contiguous structures, and while a lesser proportion (32%) were blood-born from systemic primaries).
Non-meningothelial mesenchymal tumours
There were 6 Non-meningothelial Mesenchymal tumours, comprised of 5 Hemangioblastomas (WHO grade 1) and 1 Epithelioid Hemangioendothelioma (WHO grade 3). There were 5 males and 1 female. The age range of patients with hemangioblastoma was 17-28 years with mean age of 23.25 ± 4.6 years while, the Epithelioid Hemangioblastoma patient was a 66 year old male.
Hypertrophic pachymeningitis
There were 3 patients with hypertrophic pachymeningitis aged, 13, 22 and 68 years. Two were due to Cryptococcal infection, and one was due to an inflammatory pseudo tumor. There were 2 males and 1 female.
Table III: Anatomic distribution of non-meningothelial lesions
Discussion
This study describes the demographic, clinical and histomorphologic pattern of meningeal tumours in our centre. Meningeal tumours represent 16.4% of CNS specimens evaluated in our centre in the period under review. Studies have shown that a quarter to half of intracranial tumours present in the meninges.3,6 Previous data from our center showed that meningiomas constituted 47.3% of intracranial tumours.5 Figures from other centers in Nigeria, and other sub Saharan African countries range between 26% and 42%.11-16
There was a predominance of females amongst meningioma cases, but a predominance of males amongst patients with nonmeningothelial tumors. Meningeal lesions may occur at any age even as early infancy or during fetal development.17-20 Up to 2.5% of intracranial tumours in children have been found to be meningeal based.3 Persons of all ages were affected in this study, with ages ranging between 3 years and 86 years. Children below 18years made up 7.1% of patients in this study, the greater majority of patients being adults.
The majority of meningeal tumors seen in this study were Meningiomas making up 78.6% of meningeal tumors and the commonest tumor in both adults and children. Meningiomas are the commonest meningeal lesion in both adults and children, but the clinical and pathological characteristics of meningiomas in children may differ from those of adults.3 Six meningiomas out of 110 (5.5%) in this 15-year study were seen in children below 18years of age. Salami et al. in Ibadan also found nine meningiomas in the pediatric age group amongst 166 surgically resected meningiomas (5.4%) over a 19-year period.21
Most meningiomas are histologically benign lesions (WHO grade I)and 20-25% and 1-6% of meningiomas averagely present as WHO grades II and III tumours.6,22 Findings in this study are in keeping with the above findings, though we were able to identify only 1.4% and 4.1% as grade 2 and grade 3 meningiomas respectively.
Common sites for meningiomas in the intracranial compartment include the cerebral convexities (especially parasagittal locations), the olfactory grooves, sphenoid ridges, para-/suprasellar regions, optic nerve sheath, petrous ridges, tentorium, and posterior fossa.6,23,24 observations in this study in are in keeping with the above. Studies have also shown that most spinal meningiomas occur in the thoracic region, as observed amongst our patients.6 While only 7.3% of meningiomas and 11.4% of all meningeal lesions in this study are found in the spine. Findings in a previous study from our centre show that meningeal tumors make up 20-25% of spinal tumours in our centre.25
An association between meningioma location and histological grading has been suggested, with non-skull-base meningiomas displaying more aggressive biological behavior.6,26,27 In keeping with literature, all atypical and malignant meningiomas in our cohort were found in non-skull base locations.
Molecular studies of genetic mutations associated with Meningiomas have shown mutual exclusivity between mutations like TRAF7, KLF4, AKT1, SMO and some others, and the NF2 gene mutation, with such mutations showing predilection for some locations above others.27,28 While, NF2 mutations and/or loss of chromosome 22 are predominant in meningiomas originating from the cerebral convexity and cerebellar dura and in the spinal canal.28,29,30 Comparison of skull base meningiomas, show that non-NF2 meningiomas were located on the medial skull base, whereas those on the lateral and posterior skull base harbored NF2 mutations or loss of chromosome 22.28,29,31,32 This may be linked to the difference in the embryological origins of meninges in various anatomical sites.29 The implications of these findings with regard to other meningeal lesions remain to be seen.
Meningeal metastases are found in up to 8–9% of cancer patients at post-mortem.33 Most are carcinomas and originate mostly from the breast, prostate, and lungs.7 Other common histologic entities include and germ cell tumours, head and neck region rhabdomyosarcoma, and other “small round blue cell tumours that spread from parameningeal sites to involve the adjacent dura.7,34,35 Metastatic tumours constituted the majority of Non-meningothelial tumors, forming the second commonest class of tumours in this study. They showed a male preponderance like other non-meningothelial tumors in this study. Metastatic carcinomas, secondary CNS non-hodgkin lymphomas; small round blue cell tumours; made up, 52.4%, 23.8%, and 14.3% respectively of meningeal metastases seen. The frequency of secondary CNS Lymphoma is known to vary with histological subtype, with Non-Hodgkin lymphoma being more common than Hodgkin Lymphoma.7 Secondary central nervous system lymphomas also tend to affect the leptomeninges more commonly than primary CNS lymphoma.36,37
The main mechanism for dural metastases has been found to be direct extension from overlying skull in up to 61% of cases.7 Ordinarily secondary involvement of the CNS by tumours arising in contiguous structures form a minor fraction of CNS metastases.38 However, amongst meningeal metastases, they tend to constitute a majority, as seen also in this study where they constituted 62% (13/21) of meningeal metastases. Other mechanisms of spread include haematogenous spread responsible for up to 33%; then, lymphatic spread or retrograde spread via the vertebral venous plexus,33 and from intraoperative seeding.7
Non-meningothelial mesenchymal tumors are a heterogeneous group of tumors better known in sites outside the CNS, where they occur more frequently.3 Hemangiopericytoma is the most common primary adults and children,3 but none was encountered in this study. With the exception of one Epithelioid Heamangioendothelioma in a 66year old male, the non-meningothelial mesenchymal tumors encountered in this study were all hemangioblastomas. Haemangioblastomas occur as sporadic lesions or as familial forms associated with VHL, usually in adults. The mean age at presentation of patients with hemangioblastoma in this study was 23.25 +/-4.6years, all the patients being young adults less than 30 years of age. Young age at presentation is more likely in VHL-associated tumors, as sporadic tumours tend to present 20 years later than the familial forms.39,40 However none of our patients presented with multiple lesions as is commonly seen in VHL associated Hemangioblastomas.39 Eighty percent (4/5) of our patients were males, but no gender predilection has been widely identified in literature.39
Hypertrophic Pachymeningitis is localized or diffuse inflammatory thickening of the dura presenting as mass lesions.41,42 It may result from Idiopathic, inflammatory, or infective causes like CNS tuberculosis and CNS cryptococcosis, or Rosai-Dorfman disease.1,41-44 It is an important differential diagnosis for meningeal neoplasms and may be misdiagnosed as one. Treatment depends on the cause.
Conclusion
Many of the rare histologic entities that could be encountered in the meninges, are easy to overlook as considerations in the differential diagnosis of meningeal based intracranial space occupying lesions, with perhaps the exception of the more common histologic variants of meningioma and meningeal metastases, these common entities are in turn at risk of becoming ‘waste basket’ or perhaps ‘safety net’ diagnoses for meningeal lesions. There is need for continuous evaluation and audit of histological diagnoses of meningeal lesions to avoid missing the rare entities that may occasionally be encountered. Molecular studies of genetic mutations in our population need to be done to aid understanding and prediction of biological behavior and prognosis of meningiomas and other meningeal lesions in our environment, and discover possible implications for treatment.
References
- Rezaee A, Gaillard F. Tumors of the meninges (differential). Reference article, Radiopaedia.org. (accessed on 06 Oct 2022). doi.org/10.53347/rID-35558
- Collins JM, Christoforidis GA. Meningeal Tumors. In: Newton HB. (Eds): Handbook of Neuro-Oncology Neuroimaging (Second Edition), Academic Press 2016. Pp 519-542, ISBN 9780128009451, https://doi.org/10.1016/B978-0-12-800945-1.00044-6.
- Perry A, Dehner LP. Meningeal tumors of childhood and infancy. An update and literature review. Brain Pathol. 2003 Jul;13(3):386-408. doi: 10.1111/j.1750-3639.2003.tb00038.x. PMID: 12946028; PMCID: PMC8095770.
- Demirtaş E, Erşahin Y, Yilmaz F, Mutluer S, Veral A. Intracranial meningeal tumours in childhood: a clinicopathologic study including MIB-1 immunohistochemistry. Pathol Res Pract. 2000;196(3):151-8. doi: 10.1016/S0344-0338(00)80095-3. PMID: 10729919.
- Udoh MO, Udoh DO, Aligbe JU, OluEddo AN, Ekanem VJ, Akhiwu WO, et al. Histomorphology of Intracranial Tumours in Benin-City, Nigeria. International Journal of Medical Science and Applied Biosciences. 2019; 4: 27 40.
- Perry A, Louis DN, Budka H, von Deimling A, Sahm F, Rushing EJ, et al. Meningioma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. (Eds): WHO Classification of Tumours of the Central Nervous System (Revised 4th edition). IARC; Lyon 2016. Pp 232-245.
- Lyndon D, Lansley JA, Evanson J, Krishnan AS. Dural masses: meningiomas and their mimics. Insights Imaging. 2019 Feb 6;10(1):11. doi: 10.1186/s13244-019-0697-7. PMID: 30725238; PMCID: PMC6365311.
- Bravo-Tsri AEB, Konaté I, Kouassi KPB, Acko-Ohui EV, Goulé-BI AR, Isart D, et al. Meningeal tuberculoma mimicking a brain tumor. Radiology Case Reports. Volume 16, Issue 2. 2021. Pages 284-288. ISSN 1930-0433, https://doi.org/10.1016/j.radcr.2020.11.028.
- Ghosal N, Dadlani R, Gupta K, Furtado SV, Hegde AS (2012) A clinicopathological study of diagnostically challenging meningioma mimics. J Neurooncol 106(2):339–352.
- Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY (2005) Dural metastases. J Neurooncol 75(1):57–61.
- Kanmounye US, Karekezi C, Nyalundja A, Awad A, Laeke T, Balogun J. Adult brain tumors in Sub-Saharan Africa: A scoping review. Neuro-Oncology. 2022. 10.1093/neuonc/noac098.
- Ekpene U, Ametefe M, Akoto H, Bankah P, Totimeh T, Wepeba G, Dakurah T. Pattern of intracranial tumours in a tertiary hospital in Ghana. Ghana Med J. 2018;52(2):79-83. doi: 10.4314/gmj.v52i2.3. PMID: 30662079; PMCID: PMC6326539.
- Motah M, Gams Massi D, Fouda Bekolo F, Akweseh Nju N, Ndoumbe A, Moumi M, et al. Epidemiological profile of brain tumors in Cameroon: a retrospective study. Egypt J Neurol Psychiatry Neurosurg 2021;57:126. https://doi.org/10.1186/s41983-021-00381-6.
- Jibrin P, Ibebuike K, Ado-wanka AN. Histo-pathological pattern of intracranial tumours in National Hospital, Abuja. Afri Health Sci. 2018;18(2): 281-286. https://dx.doi.org/10.4314/ahs.v18i2.12.
- Soyemi SS, Oyewole OO. Spectrum of intracranial tumours in a tertiary health care facility: our findings. The Pan African Medical Journal. 2015;20:24. doi:10.11604/pamj.2015.20.24.4935.
- Olasode BJ, Shokunbi MT, Aghadiuno PU. Intracranial neoplasms in Ibadan. Nigeria ,Moumi MEast African Medical Journal. 2000; 77 (1): 4-8 24.
- Bouvier C, Zattara-Canoni H, Daniel L, Gentet JC, Lena G, Figarella-Branger D. Cerebellar papillary meningioma in a 3-year-old boy. The usefulness of electron microscopy for diagnosis. Am J Surg Pathol 1999;23:844- 848.
- Perry A, Giannini C, Raghavan R, Scheithauer BW, Banerjee R, Margraf L, Bowers DC, Lytle RA, Newsham IF, Gutmann DH. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: A clinicopathologic study of 53 cases. J Neuropathol Exp Neurol 2001; 60:994-1003.
- Demirtas E, Ersahin Y, Yilmaz F, Mutluer S, Veral A. Intracranial meningeal tumours in childhood: A clinicopathologic study including MIB-1 immunohistochemistry. Pathol Res Pract. 2000;196:151-158.
- Amirjamshidi A, Mehrazin M, Abbassioun K. Meningiomas of the central nervous system occurring below the age of 17: report of 24 cases not associated with neurofibromatosis and review of literature. Childs Nerv Syst. 2000;16:406-416.
- Salami AA, Okunlola AI, Ajani MA, Adekanmbi AA, Balogun JA. Pediatric Meningiomas in Southwestern Nigeria: A Single-Institutional Experience. World Neurosurgery. 2019;125:e94-e97. DOI: 10.1016/j.wneu.2018.12.200. PMID: 30660895.
- Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(5):1-49. PMID:23095881.
- Mezue WC, Ohaegbulam SC, Ndubuisi CA, Chikani MC, Achebe DS. Management of intracranial meningiomas in Enugu, Nigeria. Surg Neurol Int. 2012;3:110. doi: 10.4103/2152-7806.101788. Epub 2012 Sep 28. PMID: 23087826; PMCID: PMC3475883.
- Akpa PO, Kwaghe BV, Innocent E, Otene BS, Azagaku DA, Okwudire-Ejeh I, A Retrospective Analysis of Intracranial Meningiomas in a Tertiary Health Care Facility in North Central Nigeria, American Journal of Laboratory Medicine. 2021;6(3): pp 37-41. doi: 10.11648/j.ajlm.20210603.12
- Udoh MO, Udoh DO. Histomorphologic Evaluation Of Spinal Tumors: A 14 Year Teaching Hospital Experience. Nig. Qt J. Hosp. Med. 2020;30(1-4):33-38
- Sade B, Chavlavi A, Krishnaney A, Nagel S, Choi E, Lee J H et al. World Health Organization Grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery 2007;61:1194–1198.
- Cornelius JF, Slotty PJ, Steiger HJ, Hanggi D, Polivka M, George B. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta Neurochir. 2013;155: 407–413. https://doi.org/10.1007/s00701-012-1611-y.
- Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013;339:1077–1080. https://doi.org/10.1126/science.1233009.
- Okano A, Miyawaki S, Hongo H, Dofuku S, Teranishi Y, Mitsui J, et al. Associations of pathological diagnosis and genetic abnormalities in meningiomas with the embryological origins of the meninges. Sci Rep 11, 6987 (2021). https://doi.org/10.1038/s41598-021-86298-9
- Bi WL, Abedalthagafi M, Horowitz P, Agarwalla PK, Aizer AA, Dunn GP, et al. Genomic landscape of intracranial meningiomas. J. Neurosurg. 2016;125, 525–535. https://doi.org/10.3171/2015.6.Jns15 591
- Wellenreuther R, Kraus JA, Lenartz D, Menon AG, Schramm J, Louis DN, et al Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995;146(4):827-32. PMID: 7717450; PMCID: PMC1869258.
- Youngblood MW, Duran D, Montejo JD, Li C, Omay SB, Özduman K, et al. Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg. 2019;25:1-10. doi: 10.3171/2019.8.JNS191266. Epub ahead of print. PMID: 31653806.
- Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer 2009;115(9):1947–1953
- Coene IJ, Schouwenburg PF, Voute PA, Marion J, Burgers V, Hilgers FJ. Rhabdomyosarcoma of the head and neck in children. Clin Otolaryngol Allied Sci 1992;17:291.
- Xu F, De Las Casas LE, Dobbs LJ. Primary meningeal rhabdomysarcoma in a child with hypomelanosis of Ito. Arch Pathol Lab Med 2000;124:762-765.
- Hirmiz K, Foyle A, Wilke D, Burrell S, Brownstone R, Ago C, et al. Intracranial presentation of systemic Hodgkin's disease. Leuk Lymphoma. 2004;45(8):1667-71. doi: 10.1080/10428190410001673409. PMID: 15370222.
- Montoto S, Lister TA. Secondary central nervous system lymphoma: risk factors and prophylaxis. Hematol Oncol Clin North Am 2005;19(4):751–763
- Wesseling P, von Deimling A, Aldape KD, Preusser M. Rosenblum MK, Mittelbronn M, et. al. Metastatic tumours of the CNS. In David N. Louis, Hiroko Ohgaki, Otmar D. Wiestler, Webster K. Cavenee (Eds): WHO Classification of Tumours of the Central Nervous System (Revised 4th edition). IARC; Lyon 2016. Pp 338-341
- Antonescu CR, Paulus W, Perry A, Rushing EJ, Hainfellner JA, Bouvier C, et al. Mesenchymal Non-meningothelial tumors. in: David N. Louis, Hiroko Ohgaki, Otmar D. Wiestler, Webster K. Cavenee (Eds): WHO Classification of Tumours of the Central Nervous System (Revised 4th edition). IARC; Lyon 2016. Pp 248- 257
- Maher ER, Yates JR, Ferguson-Smith MA. Statistical analysis of the two stage mutation model in von Hippel-Lindau disease, and in sporadic cerebellar haemangioblastoma and renal cell carcinoma. J Med Genet. 1990: 27(5):311-4. PMID:2352258
- Weerakkody Y, Sharma R. Hypertrophic pachymeningitis. Reference article, Radiopaedia.org. (accessed on 12 Nov 2022) https://doi.org/10.53347/rID-10824
- Abrantes F, Moraes M, Rezende Filho F, Pedroso J, Barsottini O. A Clinical Approach to Hypertrophic Pachymeningitis. Arq Neuro-Psiquiatr. 2020;78(12):797-804. doi:10.1590/0004-282x20200073
- Friedman DP, Flanders AE. Enhanced MR imaging of hypertrophic pachymeningitis. AJR Am J Roentgenol. 1997;169 (5): 1425-8.
- Jones SE, Belsley NA, Mcloud TC et-al. Rheumatoid meningitis: radiologic and pathologic correlation. AJR Am J Roentgenol. 2006;186 (4): 1181-3. doi:10.2214/AJR.05.0859