Prevalence, presentation, risk factors and awareness of oral cancer: A systematic review of the Nigerian experience
Uchenna P. Egbunah, BDS, MD
Abstract
Background: Oral cancers are malignant neoplasia of the lip and/or oral cavity that represent a major part of head and neck carcinomas (HNCs) along with laryngeal and pharyngeal carcinomas. Although oral cancers can be considered a health burden, available evidence on the actual burden in Nigeria is sparse.
Aims: The aim of this review was to determine the prevalence, presentation, risk factors, and awareness of oral cancer in different geopolitical zones in Nigeria.
Methods: Systematic literature searches were conducted in PubMed (NLM), Cochrane, Ovid Medline, OpenGrey and Google scholar databases, to identify publications on the prevalence, presentation, risk factors, and awareness of oral cancer in different geo-political zones in Nigeria.
Results: Oral cancer was found to be the most common form of HNCs in several of the included studies. Although reports from included studies varied across geopolitical zones in Nigeria, generally, oral cancers were most found in males, between the 5th and 6th decade, and the tongue, palate, maxilla, and mandible were common sites. In addition, a suboptimal level of awareness of oral cancer was reported by the majority of reviewed studies.
Conclusion: This review showed variations in prevalence, presentation, and risk factors of oral cancer across different geopolitical zones in Nigeria and highlighted the low level of knowledge and awareness of oral cancer among the Nigerian population.
Keywords: oral cancer; prevalence; presentation; risk factors; awareness; systematic review; Nigeria
Introduction
Oral cancers are malignant neoplasia of the lip and/or oral cavity that represent a major part of head and neck carcinomas (HNCs) along with laryngeal and pharyngeal carcinomas. They remain a health burden as their incidence ranks sixth globally amongst all cancers, accounting for approximately 5% of all malignant tumors.1,2 Majority (90%) of oral cavity carcinomas originate histologically from squamous cells, therefore, oral cancers are traditionally defined as oral squamous cell carcinomas (OSCC).3,4 They are associated with low five-year survival rate especially in underdeveloped and developing countries5 and they represent a major cause of morbidity and mortality worldwide.5,6
Nigeria is a developing country in Western Africa with an estimated population of 225 million persons (2023) spread across a land area of 923,768.64 square kilometers.7 The country is made up of six geopolitical zones including Northcentral, Northeast, Northwest, Southeast, Southsouth and Southwest Nigeria, and although the reason is unclear, previous studies have suggested a varied prevalence and presentation among these geopolitical zones within the Nigerian population.8 Available evidence on the actual burden of oral cancer in Nigeria is sparse.9 This is further highlighted by the low number of oral cancer related publications from Nigeria seen in various global cancer registries.5 In addition, majority of the oral cancer related studies in Nigeria are hospital-centered and not population-centered which may not give an accurate representation of the oral cancer burden in Nigeria.9,10
The World Health Organization (W.H.O) has reported an increased prevalence of cancer-related deaths among underdeveloped countries.9 Although Nigeria is a developing country with 11 cancer registries located in teaching hospitals across the country, majority of these centers are poorly funded which may result in unfavorable oral cancer management outcomes.5 Cancer-related deaths are often times associated with the stage of the lesion at diagnosis and intervention; early stage lesions present with better prognosis compared to their late stage counterparts.11,12
In Nigeria, oral cancer is frequently diagnosed in the late stage as most patients present later in the course of the disease.13,14 Several studies4,5,13–15 have attributed this negative health-related behavior to financial challenges, lack of awareness and traditional beliefs. These studies4,5,13–15 led to the proposition and implementation of oral cancer awareness campaigns and advocacies across various communities in Nigeria over the past decade, the effects of which is sparsely documented, and requires further investigation. With this in view, this paper aimed to review the prevalence, presentation, risk factors, and awareness of oral cancer in different geopolitical zones in Nigeria, and proffer solutions to problematic trends identified.
Methodology
Eligibility criteria: In order to ensure a thorough review of all available information, case-control, retrospective, cohorts, cross-sectional and experimental studies were included to allow for review of a wide collection of information and high level of evidence. Case reports, case series and review articles were excluded. Studies on HNCs were also excluded from the review. However, in cases where data for oral cancers could be extracted from HNCs; extractable data were included in the review.
The initial search was done to only include studies published up to December 2023. However, independent searches yielded studies published after this date. This was done to ensure review of more recent and up to date information. The included studies had to report on the prevalence of oral cancer in a Nigerian population, the presentation of oral cancer, identified risk factors, and/or awareness of oral cancer in a Nigerian population.
Only articles written in English or with English language translations were considered for the review. There were no other publication conditions. Studies included were of different study designs, published in different parts of Nigeria to allow for heterogeneity of source of information for the review.
Nigeria can be divided into six geopolitical zones: Northcentral, Northeast, Northwest, Southeast, Southsouth and Southwest, and oral cancers have been shown to have varying distribution patterns across these zones. Included studies were divided according to the geopolitical zones in Nigeria to allow for comparability between zones in Nigeria. Multicentered studies with included participants from different geopolitical zones in Nigeria were also included, stating all the geopolitical zones represented in the study.
Information sources: Computerized systematic literature searches were conducted in PubMed (NLM), Cochrane, Ovid Medline, OpenGrey and Google scholar databases to identify publications on prevalence, presentation, risk factors, and awareness of oral cancer in Nigeria published till December 2023. Hand searching of the reference section of selected studies and purposeful online searches up to November 2024 were also done to identify additional eligible studies.
Search strategy: The search strategy for PubMed involved the use of the following keywords and Boolean operators: oral cancer OR oral carcinoma OR oral squamous cell carcinoma OR head and neck cancers AND prevalence OR epidemiology OR distribution AND etiological factors OR risk factors AND presentation OR clinical presentation OR histologic presentation AND awareness OR knowledge AND Nigeria. Similar search strategies were used for other database searches, and the inclusion criteria used for screening were articles written in English language by Nigerian authors about the Nigerian population.
Study selection: A total of 137 article abstracts were identified after inclusion of all keywords. The identified studies were screened for eligibility. For studies appearing to meet the inclusion criteria or for which there were insufficient data in the abstract to make a clear decision, full study reports were obtained. Full reports were also assessed to determine whether the studies met all the criteria and to eliminate duplicate studies. Only 29 articles were identified as meeting all inclusion criteria and were included in the study. Details of the study selection process are shown in Figure 1.
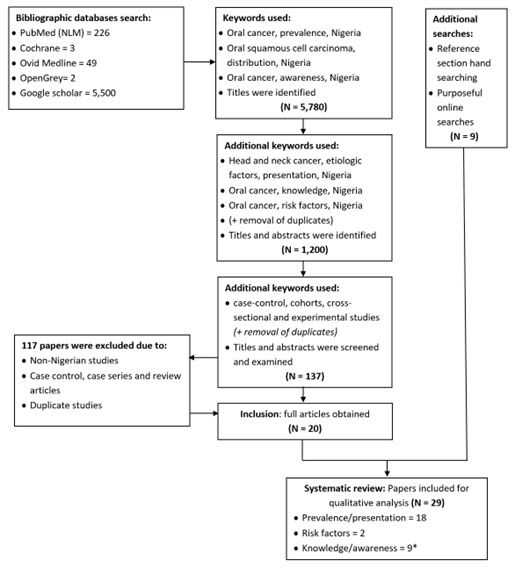
Figure 1: Flow chart of the study search strategy and selection process according to PRISMA16
Data extraction: Data extraction was done if the paper provided original data on oral cancer in Nigeria in accordance with the aim of this review and when available, qualitative and quantitative analyses of study data were extracted and reported. The data extraction forms were piloted on several papers and modified as required before use.
Data items: For included studies, the following data were recorded (where available): Author’s name, year of publication, study design, geopolitical zone in Nigeria, prevalence of oral cancer, age, sex, site and histopathology of oral cancer in Nigeria.
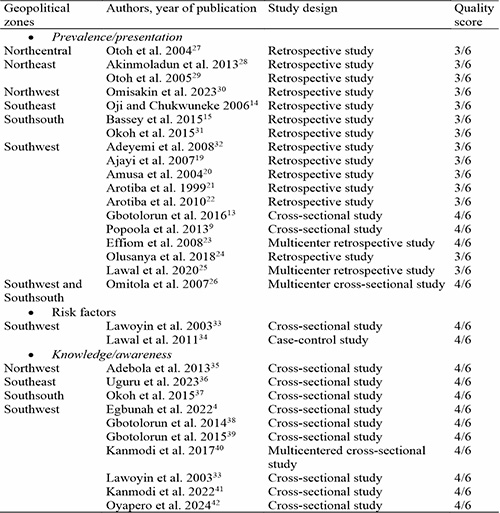
Methodological quality assessment: The quality of included studies was assessed using a modified version of the Newcastle-Ottawa scale (NOS)17 adopted from Barbosa et al, 2017.18 The NOS items used (where available) were study design, sample selection criteria (case definition, patient representativeness and patient selection process) and oral cancer evaluation criteria (diagnostic processes and sample loss for longitudinal studies) for oral cancer prevalence/presentation studies. Study design, sampling method and sample size determination were used for knowledge/awareness studies. The scale scores ranged from 0 to 6 with 0, 1, 2 representing low quality, 3, 4 representing moderate quality, and 5, 6 representing high quality.
Results
Included studies
The review process yielded 29 full articles that met all inclusion criteria: 18 studies9,13–15,19–32 on prevalence/ presentation of oral cancer, two studies33,34 on risk factors of oral cancer, and nine studies4,35–42 of knowledge/ awareness of oral cancer. The study by Lawoyin et al. 200333 was included in the review for risk factors and knowledge/awareness of oral cancer. The PRISMA flow chart for this review can be seen in Figure 1 and details of included studies can be found in Table I.
Table I: Table of included studies
Quality assessment
The quality of studies was evaluated using the NOS scale adopted from Barbosa et al, 2017.18 All included studies were of moderate or intermediate quality according to the NOS scale. No low-quality study was identified. The quality assessment result for each study is itemized in Table I.
Prevalence
Oral cancers are among the most common cancers worldwide, with approximately 442,760 incident cases and 241,418 deaths reported in the last worldwide oral cancer census.43,44 There are geographical variations in the incidence of oral cancers, with increase among men and women in some European and African countries, stabilization in certain Asian countries, and decrease in Canada and USA.45,46
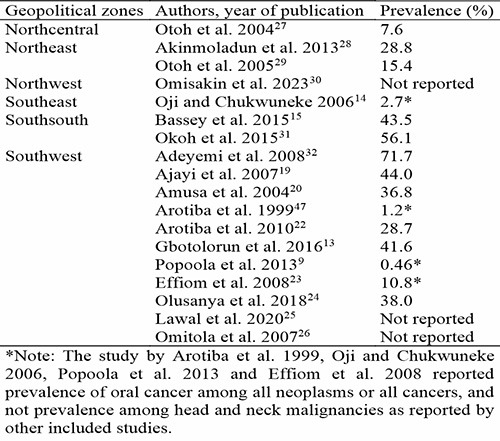
Several epidemiological studies have been conducted on oral cancer in different geopolitical zones in Nigeria. North-Central Nigeria reported that OSCC constitute 7.6% of all HNCs27 while North-East Nigeria reported 19.5% of all HNCs.28 OSCC was reported to account for 2.7% of all cancer cases in South-East Nigeria,14 18.7% – 25.1% in South-South Nigeria15,31 and 9.7 – 18% in South-West Nigeria.47,48 Omitola et al. 201726 conducted a prospective multicentered study involving four oral cancer healthcare centers across two geopolitical zones; Southsouth and Southwest. Results of this study showed that oral cancer was significantly more prevalent in the Southsouth region (59.2%) compared to the Southwest region (40.8%).
Survival following a diagnosis of OSCC remains poor with overall five-year survival around 68%.49 Only limited improvements have been recorded since the late 1980s.49 The results of these previous studies suggest regional variations in prevalence of oral cancers across geopolitical zones in Nigeria arising from the varying lifestyle and cultural practices of these regions. In addition, studies50 have pointed to an increase in incidence and mortality rates of oral cancers especially among the younger age group, which may be due to lifestyle changes and/or increased awareness and reporting of the condition. Table II shows prevalence of oral cancer among HNCs across different geopolitical zones in Nigeria.
Table II: Prevalence of oral cancer across geopolitical zones in Nigeria
Age distribution
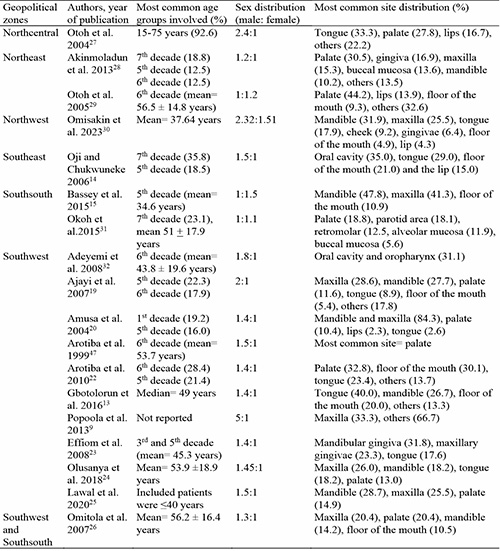
Although oral cancer can occur at any age, it is commonly considered as a disease of the middle age and elderly.51 Previous studies51,52 have suggested that the increased incidence of oral cancer with increasing age may be due to the increased reactions of free radicals in the body with age. In addition, these studies51,52 suggest that cancer surveillance by immune cells reduce with age which results in a diminished ability of the immune system to eliminate damaged and mutated cells. Oral cancers have also been associated with harmful social habits such as cigarette smoking and alcohol consumption.4,34 These habits have a deterministic effect on the occurrence of several types of cancer including oral cancers.34 The effects of these habits buildup over time, and may be responsible for the oral cancer eventuality occurring in an older age group. In Nigeria, a recent study by Gbotolorun et al. 201613 showed that the peak age of incidence of oral cancer was in the 5th decade. In addition to the 5th decade, similar studies14,15,19,28,53 conducted in the Nigerian population have reported the 6th and 7th decade as the most common age group involved. The study by Otoh et al. 200427 reported a wide age range of 15-75 years. The multicentered study by Omitola et al. 201726 reported a higher mean age distribution in the Southsouth of 65.4 years compared to a mean age of 54.4 years seen in the Southwest region. The study by Amusa et al. 200420 was the only study to report the 1st decade as the most prevalent age range for incidence of oral cancer. This discrepancy, however, may be due to the addition of all HNCs including cancers in the oral cavity, neck, thyroid, esophagus, and paranasal sinuses (Table III).
Table III: Age, sex, and site distribution of oral cancer across geopolitical zones in Nigeria.
Sex distribution
Oral cancers have been reported to be two to three times more common in males than females.51 This is majorly attributed to social habits such as cigarette smoking, alcohol consumption, betel nut chewing, etc. that are more commonly practiced by males than females. Recent studies, however, have reported a reduction in the male predilection for oral cancer, stating that the practice of these harmful social habits are no longer peculiar to males.54 This is similar to reports from majority of previous Nigerian studies.9,13,19,22,28 The study by Popoola et al. 20139 reported a male to female ratio as high as 5:1. Other Nigerian studies13,14,22,27,53 reported reduction in male preponderance ranging from 2.4 to 1.1:1 male to female ratio. The multicentered study by Omitola et al. 201726 reported a relatively even male to female ratioin the Southwest region while a male preponderance was seen in the Southsouth region with a male to female ratio of 1.5:1.
The study by Otoh et al. 200529 conducted in Northeast Nigeria reported a slight female preponderance for oral cancer. A similar result was seen in Southsouth Nigeria by Bassey et al. 201515 who reported a male to female ratio of 1:1.5 (Table III). These discrepancies highlight the variations of oral cancer presentation in different geopolitical zones in Nigeria. In addition, the increased female predilection of oral cancer reported in these two studies15,29 corroborate the findings of Scully, 201354 who reported a reduction in male predilection for oral cancer due to the universal practice of harmful social habits, no longer peculiar to men.
Site distribution
The tongue is the most common site for oral cancer in the U.S.55 Some studies have reported that the floor of the mouth and the lip are most common in patients who consume alcohol regularly and in light-skinned patients respectively.55 In Northcentral Nigeria, the tongue was the most common site for oral cancer.27 Also, the oral cavity and tongue were the most common site for oral cancer in Southeast Nigeria,14 while the palate was the most common site for oral cancer in Northeast Nigeria.28,29 Studies from Southsouth and Southwest Nigeria reported varied site predilection which included maxilla, mandible, palate, and tongue as most common sites for oral cancer9,13,15,19,20,22,53 (Table III). Figures 2 – 4 shows oral cancer in different locations in the oral cavity.
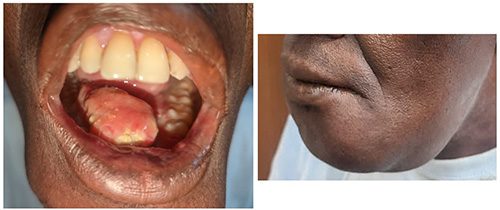
Figure 2: Oral cancer of the floor of the mouth raising and displacing the tongue and extending to involve the mandible (left: intraoral, right: extraoral). (Image credit: Department of Oral and Maxillofacial Surgery, Lagos University Teaching Hospital, Nigeria)
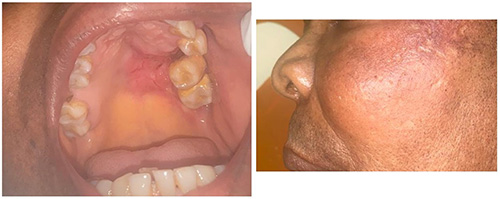
Figure 3: Oral cancer of the palate showing non-healing ulcer, extraction socket indicating tooth loss, and molar teeth displacement due to bone destruction by cancer (left: intraoral, right: extraoral). (Image credit: Department of Oral and Maxillofacial Surgery, Lagos University Teaching Hospital, Nigeria)
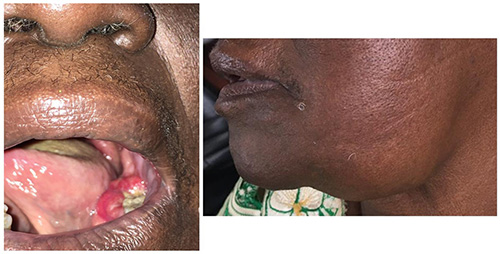
Figure 4: Oral cancer of the mandible showing large inflamed non-healing ulcer originating from the molar and paramolar region (left: intraoral, right: extraoral). (Image credit: Department of Oral and Maxillofacial Surgery, Lagos University Teaching Hospital, Nigeria)
Histopathological types of oral cancer
Oral cancers may develop from epithelial (carcinomas), mesenchymal (sarcomas), or hematolymphoid tissues (lymphomas, leukemias, plasmacytomas).56 However, the most common histopathologic presentation of oral cancer is the OSCC which originates histologically from squamous cells.3 Reports from developed countries state that the OSCC make up about 90% of all oral cavity carcinomas.3 Similar to these reports,3 in North central Nigeria, Otoh et al. 200427 reported that among all diagnosed HNCs, 81.5% were carcinomas, 16.7% were sarcomas, and 1.8% were lymphomas, and OSCC represented 84.1% of diagnosed carcinomas. Similar results were seen in other geopolitical zones in Nigeria. In Northeast Nigeria, carcinomas (majorly OSCC) represented majority of HNCs28,29 with Akinmoladun et al. 201328 reporting a prevalence of 81.3%. From the Southeast, Oji and Chukwuneke 200614 reported that 96% of all invasive carcinomas were OSCCs. Majority of reports from Southwest Nigeria reported a prevalence of OSCC of 60-70%.19,22,32 Ajayi et al. 200719 reported a prevalence of 63% of all HNCs, Arotiba et al. 201022 reported 66.4%, and Adeyemi et al. 200832 reported 66.7%. Gbotolorun et al. 201613 reported a prevalence of 41.7% of all HNCs, which was the most common histologic type reported, but was lower than other reports from the region. Similarly, in Southsouth Nigeria, although OSCC were reported as the most common histopathologic type of HNCs, its prevalence was not as high as reported in other geopolitical zones in Nigeria. Bassey et al. 201515 reported that OSCC was the most common with a prevalence of 43.5%, followed by Burkitt lymphoma 17.4%, estrogenic sarcoma 13.0%, rhabdomyosarcoma 8.7%, adenoid cystic carcinoma 6.5%, mucoepidermoid carcinoma 4.3%, and non-Hodgkins lymphoma 2.2% in that order. Also, Okoh et al. 201531 reported majority of oral cancers as OSCC (58.6%), with acinic cell (8.6%) and adenocystic carcinomas (8.1%) as second and third respectively.
Risk factors of oral cancer
The etiology of oral cancer is multifactorial involving multiple steps.57 However, there is substantial evidence that precancerous habits such as tobacco use, alcohol consumption, betel quid and kola nut chewing, exposure to carcinogens in the workplace, excessive sun exposure, poor diet, vitamin deficiency, viral infections and sexual behaviors resulting in Human Papilloma Virus (HPV) infection are significant risk factors in the etiology of OSCC.49,58–61
Tobacco has been proven to increase the risk of several cancers including oral cancer, and it has a synergistic effect with alcohol consumption. Studies62,63 have shown that the risk for developing oral cancer is increased three times in patients who smoke cigarettes, and the risk of a nonsmoker developing oral cancer increases by 87% when exposed to consistent secondhand smoke. In addition, Marron et al. 201064 reported that the risk of developing oral cancer reduces by 35% if a patient quits smoking for at least 4 years. The tobacco and the smoke from the cigarette results in hypoxia and weakened immunity of the oral environment resulting in outcomes such as gingivitis, periodontitis, and oral cancer.55 In Nigeria, tobacco has been associated with the development of oral cancer. Otoh et al. 200427 in Northcentral Nigeria reported an association between cigarette smoking and development of oral cancer. Another study65 from Northcentral Nigeria reported a synergistic effect and a significant association between tobacco smoking, alcohol consumption and oral cancer. Similarly, Lawal et al. 201134 in Southwest Nigeria reported a 4.05 increased risk in oral cancer development with tobacco use.
Kola nut chewing and tobacco chewing have also been reported to be significantly associated with oral cancer in the Nigerian population. In Northcentral Nigeria, kola nut and tobacco chewing were associated with development of oral cancer.27 In addition, Odukoya et al. 199066 in Southwest Nigeria reported that kola nuts promote palatal keratinization in cigarette smokers serving as synergistic co-carcinogen, promoting the development of OSCC. Unhealthy diets have also been associated with the development of oral cancer in Nigerians. In Southwest Nigeria, Lawal et al. 201134 reported that the odds of developing oral cancer was three times more in patients who did not consume fruits and vegetables regularly. Similarly, a study65 in Northcentral Nigeria reported that patients with oral cancer had significantly lower levels of antioxidant vitamins A, C and E compared to healthy controls with p<0.05 for all tested associations.
Not all reports out of Nigeria agreed with current trends. Two studies33,47 from Southwest Nigeria showed no association between oral cancer and known precancerous habits like tobacco and alcohol. They33,47 suggested that other predisposing factors like genetics, nutrition, and infection may play more important roles in oral cancer development in their environment. Similarly, Oji and Chukwuneke in Southeast Nigeria reported that majority of patients with oral cancer in their health center gave no positive history of tobacco use and alcohol consumption.14 They14 postulated other risk factors such as poor education, low socioeconomic class, poverty, malnutrition, and poor oral hygiene as major causes of oral cancer in their environment.
Knowledge and awareness of oral cancer
Oral cancer is one of the most lethal diagnoses encountered in dental and maxillofacial surgery.13 In Nigeria, It is often encountered at a late stage due to late patient presentation resulting in poor prognosis.13,47 This is often attributed to low socioeconomic class, illiteracy, healthcare inaccessibility, harmful beliefs and stereotypes, utilization of traditional, and untested healthcare methods.34,65 A study done on the knowledge of oral cancer among adults in a rural community in Southwest Nigeria showed that over 90% of respondents had low knowledge of the general features of oral cancer, 61.7% had low knowledge of its risk factors, 71.7% had low knowledge of its treatment options and 75.3% had low overall knowledge of oral cancer.4 The study also showed that increased knowledge was associated with higher level of education and increased utilization of healthcare facilities (p<0.05 for both associations).4 A similar study in Northwest Nigeria among traditional healers and herbalists reported that majority (66.7%) of traditional healers had never heard of oral cancer.67 Knowledge of oral cancer was also significantly associated with level of education; traditional healers with low level of education exhibited low levels of knowledge of oral cancer.67 This is particularly concerning because previous studies34,65 have reported that a good number of Nigerians frequent these traditional healers and herbalists, and if these healers do not have adequate knowledge of oral cancer, it further results is poor management, late presentation, and overall poor prognosis of oral cancer in the Nigerian population. Recent reports from Southeast Nigeria among military personnel showed only 15.3% had knowledge of oral cancer, and less than half of them could identify risk factors of oral cancer such as cigarette smoking and alcohol consumption.36 The study also showed that majority of military personnel believed that oral cancer was caused by a supernatural phenomenon, further highlighting the deficiencies in knowledge.36 A study by Kanmodi et al. 202241 assessed knowledge of HNCs and oral cancer among secondary school students in Nigeria. The study41 included respondents from all geopolitical zones in Nigeria except Southeast Nigeria. Only 31.2% of them had knowledge of HNCs, and more than half of them had below average scores in their knowledge assessment tests.41 In addition, a low (32.4%) amount of respondents had received either formal or informal education on oral cancer, and over 80% of respondents indicated an interest in knowing more about HNCs and oral cancers, indicating a lack of awareness of oral cancer among secondary school students in Nigeria. In Southsouth Nigeria, a study on knowledge of oral cancer among graduating dental students showed that although all participants had knowledge of oral cancer, only about 23% of students had high scores in their knowledge of oral cancers and risk factors test,37 corroborating results from other Nigerian studies. The lack of significantly high knowledge of oral cancer indicates the need for improved awareness to improve outcomes of oral cancer in the Nigerian population.
Discussion
The prevalence of oral cancer reported in included studies in this review highlights variations in prevalence across different geopolitical zones in Nigeria which is like previous reports both within5,51 and outside45,46 Nigeria. The differences may be due to differences in cultural and lifestyle practices and may also be due to reporting bias as no study on prevalence of oral cancer was found from Northwest Nigeria. Studies45,46 from developed countries have reported a reduction in the incidence of oral cancer primarily due to increased oral cancer surveillance. This review, however, points to an increase in incidence and mortality of oral cancer which may be due to increased risk factors, failed oral cancer surveillance, or due to reporting bias, as more studies on oral cancer have been published in the past decade compared to previous decades. Irrespective of the reason, it’s clear that the effects of oral cancer awareness programs conducted over the past decade have had limited effects on oral cancer outcomes, which is a cause for concern.
Also highlighted in this study is the increased presentation of oral cancer among lower age groups. Oral cancer is particularly known to be a disease of middle age and elderly, however, studies by Amusa et al. 200420 and Otoh et al. 200427 reported increased involvement of patients in their first and second year of life respectively. Sex distribution shows a predilection for the male sex, however, like studies in developed countries,45,46 this review shows narrowing between the male and female sex in incidence of oral cancer. Variations in site predilection have been reported in both developed45,46 and developing countries.5,51 In this review, variations in site predilection may be due to differences in social habits across geopolitical zones in Nigeria. Studies from Northcentral Nigeria27,65 reported increased cigarette smoking and alcohol consumption habits compared to reports from Southwest33,47 and Southeast Nigeria14 that reported genetics, nutrition, and infection as more important roles in oral cancer development compared to tobacco and alcohol.
This review also brings to the fore the low level of knowledge on oral cancer among the general population in Nigeria. Knowledge of oral cancer was seen to be significantly associated with the level of education.4 Public policies for free and affordable education up to secondary school level is therefore recommended, as this will significantly improve oral cancer outcomes and overall healthcare in the Nigerian population This review also highlighted the need for effective oral cancer education and surveillance among educated individuals, as low levels of knowledge on oral cancer was seen in secondary school students and military personnels, both of whom had received significant formal education.37,41
Although oral cancer awareness programs have been recommended and instituted in the past across Nigeria,4,5,13–15 this review highlights a lack of effectiveness in current practices. It is therefore recommended that stronger oral cancer awareness and advocacy programs be instituted across all levels of government in all geopolitical zones in Nigeria. Aggressive oral cancer training and surveillance including regular oral health checks, early recognition of symptoms, knowledge on risk factors, and dangers associated with poor management and late presentation should be implemented across all geopolitical zones in Nigeria. In the hospital setting, medical and paramedical practitioners should be trained in methods for early detection of oral cancer as this will improve the prognosis and overall outcome of oral cancer in Nigeria. The results of this review may also inform investigators on gaps requiring future research such as the need for convenient and readily available screening tools for early detection of oral cancer in Nigeria, as well as further investigation on the molecular events associated with oral cancer in Nigeria.
Conclusion
Oral cancers are the most common forms of head and neck cancers, and they represent a public health burden in Nigeria. This review showed variations in prevalence, age predilection, sex predilection, site predilection, histopathology, and risk factors of oral cancer across different geopolitical zones in Nigeria. In addition, this review highlighted the low level of knowledge and awareness of oral cancer among the Nigerian population, particularly seen in people with low level of education.
There is therefore a need for increased awareness of oral cancer among Nigerians, as well as improved advocacy, preventive care, surveillance, and screening methods for oral cancer to aid overall outcomes of oral cancer in Nigeria. In addition, to reduce the prevalence of oral cancer in Nigeria, school-based and community-based education programs on oral cancer risk factors, public education-based campaigns on awareness and early detection of oral cancer, and early and affordable clinical interventions by medical and dental practitioners are recommended.
Conflict of interest
The authors declare no conflict of interest.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
Nil.
References
- Slootweg PJ, Eveson JW. Tumours of the Oral Cavity and Oropharynx. In: L B, JW E, P R, D S, eds. Pathology and Genetics of Head and Neck Tumours. 3rd ed. International agency for Research on Cancer, World Health Organization; 2005:166-175. Accessed June 25, 2020. https://screening.iarc.fr/doc/BB9.pdf
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. doi:10.3322/caac.21262
- Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44(1):10-22. doi:10.1016/j.oraloncology.2007.06.011
- Egbunah UP, Uti OG, Sofola OO. Knowledge of Oral Cancer and Its Effect on Oral Healthcare Practices and Precancerous Habits in a Rural Community in Nigeria. Int J Med Pharm Res. 2022;3(1):01-12. doi:10.5281/zenodo.7361306
- Okoh M, Okoh DS. Oral Cancer- The Nigerian Perspective. J Mol Biomark Diagn. 2017;08(06). doi:10.4172/2155-9929.1000369
- Uti OG, Fashina AA. Oral cancer education in dental schools: knowledge and experience of Nigerian undergraduate students. J Dent Educ. 2006;70(6):676-680.
- Worldometers. Nigeria Population - Worldometers.
- Okorie PN, Ademowo GO, Saka Y, et al. Lymphatic filariasis in Nigeria; micro-stratification overlap mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl Trop Dis. 2013;7(9):e2416. doi:10.1371/journal.pntd.0002416
- Popoola A., Omodele FO, Oludara MA, Ibrahim NA, Igwilo AI, Makanjuola SBL. Prevalence and Pattern of Cancers among Adults Attending a Tertiary Health Institution in Lagos, Nigeria. IOSR J Dent Med Sci. 2013;6(3):68-73. doi:10.9790/0853-0636873
- da Lilly-Tariah OB, Somefun AO, Adeyemo WL. Current evidence on the burden of head and neck cancers in Nigeria. Head Neck Oncol. 2009;1:14. doi:10.1186/1758-3284-1-14
- Kurokawa H, Zhang M, Matsumoto S, et al. The high prognostic value of the histologic grade at the deep invasive front of tongue squamous cell carcinoma. J oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2005;34(6):329-333. doi:10.1111/j.1600-0714.2005.00244.x
- Silveira EJD da, Godoy GP, Lins RDA, et al. Correlation of clinical, histological, and cytokeratin profiles of squamous cell carcinoma of the oral tongue with prognosis. Int J Surg Pathol. 2007;15(4):376-383. doi:10.1177/1066896907304992
- Gbotolorun OM, Emeka CI, Effiom O, Adewole RA, Ayodele AS. An Audit of Malignant Oro-facial Tumors Presenting at a Tertiary Hospital in Lagos. Ann Med Health Sci Res. 2016;6(2):133-136. doi:10.4103/2141-9248.181840
- Oji C, Chukwuneke FN. Oral cancer in Enugu, Nigeria, 1998-2003. Br J Oral Maxillofac Surg. 2007;45(4):298-301. doi:10.1016/j.bjoms.2006.09.001
- Bassey GO, Osunde OD, Anyanechi CE. Analysis of 46 cases of malignant jaw tumours in Calabar, Nigeria. Niger Med J. 2015;56(4):240-243. doi:10.4103/0300-1652.169696
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
- Wells G, Shea B, Robertson J, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale ( NOS ) for Assessing the Quality of Nonrandomized Studies in Meta- Analysis Bias and Confounding Newcastle-Ottowa Scale. Ottawa Hosp Res Inst. Published online 2012. http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf
- Barbosa KGN, de Macedo Bernardino Í, d’Avila S, Ferreira EF e., Ferreira RC. Systematic review and meta-analysis to determine the proportion of maxillofacial trauma resulting from different etiologies among children and adolescents. Oral Maxillofac Surg. 2017;21(2):131-145. doi:10.1007/s10006-017-0610-9
- Ajayi OF, Adeyemo WL, Ladeinde AL, et al. Primary malignant neoplasms of orofacial origin: a retrospective review of 256 cases in a Nigerian tertiary hospital. Int J Oral Maxillofac Surg. 2007;36(5):403-408. doi:10.1016/j.ijom.2007.01.007
- Amusa YB, Olabanji JK, Ogundipe O V., et al. Pattern of head and neck malignant tumours in a Nigerian teaching hospital - A ten year review. West Afr J Med. 2004;23(4):280-285. doi:10.4314/wajm.v23i4.28141
- Arotiba JT, Obiechina AE, Fasola OA, Fawole OI, Ajagbe HA. Oral squamous cell carcinoma: a review of 246 Nigerian cases. Afr J Med Med Sci. 1999;28(3-4):141-144.
- Arotiba GT, Arotiba JT, Taiwo AO. Biologic, anatomic and clinical considerations in the management of the classic intraosseous ameloblastoma of the jaws. Nig Q J Hosp Med. 2010;20(2):55-63. doi:10.4314/nqjhm.v20i2.58026
- Effiom OA, Adeyemo WL, Omitola OG, Ajayi OF, Emmanuel MM, Gbotolorun OM. Oral squamous cell carcinoma: a clinicopathologic review of 233 cases in Lagos, Nigeria. J oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2008;66(8):1595-1599. doi:10.1016/j.joms.2007.12.025
- Olusanya A, Adisa A, Aladelusi T, et al. Orofacial Cancers: Pattern and Management in Ibadan, Nigeria. Med J Zambia. 2018;45(4):179-188.
- Lawal A., Adisa A., Effiom O. Oral squamous cell carcinoma in patients less than 40 years in a Nigerian population. African J Oral Heal. 2020;9(2):40-45. doi:10.4314/ajoh.v9i2.4
- Omitola OG, Soyele OO, Sigbeku O, et al. A multi-centre evaluation of oral cancer in southern and Western Nigeria: An African oral pathology research consortium initiative. Pan Afr Med J. 2017;28:1-10. doi:10.11604/pamj.2017.28.64.13089
- Otoh E, Johnson N, Mandong B, Danfillo I. Pattern of oral cancers in the North Central zone of Nigeria. African J Oral Heal. 2004;1(1):47-53. doi:10.4314/ajoh.v1i1.31305
- Akinmoladun VI, Akintububo OB, Adisa AO, Ojo EO, Ayuba D. Evaluation of the histopathology of orofacial lesions in a North-East Nigerian tertiary centre. Ann Afr Med. 2013;12(2):105-109. doi:10.4103/1596-3519.112401
- Otoh EC, Johnson NW, Olasoji HO, Danfillo IS, Adeleke OA. Intra-oral carcinomas in Maiduguri, north-eastern Nigeria. Oral Dis. 2005;11(6):379-385. doi:10.1111/j.1601-0825.2005.01134.x
- Omisakin O., Dekah I, Ogunsina A, Zubairu R, Ayuba G. Challenges in the Management of Oral Cancers : An Audit of Cases Treated in a Teaching Hospital North-. Niger J Med Dent Educ. 2023;5(2):48-53.
- Okoh DS, Orikpete E V, Omoregie OF, Ojo MA. A study of the clinicopathologic patterns of Orofacial carcinomas in a Nigerian population. Afr J Oral Maxillofac Pathol Med. 2015;1(2):10-17.
- Adeyemi BF, Adekunle L V, Kolude BM, Akang EEU, Lawoyin JO. Head and neck cancer--a clinicopathological study in a tertiary care center. J Natl Med Assoc. 2008;100(6):690-697. doi:10.1016/s0027-9684(15)31343-2
- Lawoyin JO, Aderinokun GA, Kolude B, Adekoya SM, Ogundipe OT. Oral cancer awareness and prevalence of risk behaviours among dental patients in South-western Nigeria. Afr J Med Med Sci. 2003;32(2):203-207.
- Lawal A, Kolude B, Adeyemi BF, Lawoyin J, Akang E. Social profile and habits of oral cancer patients in Ibadan. Afr J Med Med Sci. 2011;40(3):247-251.
- Adebola RA, Bamgbose BO, Adeoye JB, Amole TG. Awareness of Oral Cancer in a Northwestern Nigerian State: Assessing the Knowledge, Opinion, and Practice of Traditional Healers and Herbalists. J Oral Oncol. 2013;2013:1-7. doi:10.1155/2013/263150
- Uguru C, Chukwubuzor O, Otakhoigbogie U, Ogu U, Uguru N. Awareness and knowledge of risk factors associated with oral cancer among military personnel in Nigeria. Niger J Clin Pract. 2023;26(1):73. doi:10.4103/njcp.njcp_322_22
- Okoh M, Enabulele J. Knowledge and practices regarding oral cancer among graduating dental students. Indian J Oral Sci. 2015;6(1):14. doi:10.4103/0976-6944.154602
- Gbotolorun OM, Ayodele ASO, Olojede ACO, Adamson OO, Emeka CI, Amao AT. Knowledge and screening practices for oral cancers amongst general dental practitioners in Lagos, Nigeria. African J Biomed Res. 2014;17(2):69-73.
- Gbotolorun O, Eweka O, Lawal A, Fadeyibi O, Emeka C. Knowledge, opinions, and practices about oral cancer among general medical practitioners in Lagos, Nigeria. J Oral Res Rev. 2015;7(1):6. doi:10.4103/2249-4987.160169
- Kanmodi K, Amoo B, Sopeju A, Adeniyi O. Oral Cancer and Oral Sex: Awareness and Practice among Nursing Students in Ibadan Metropolis, Nigeria. Asian J Med Heal. 2017;2(4):1-8. doi:10.9734/ajmah/2017/29935
- Kanmodi KK, Fagbule OF, Ogbeide ME, et al. Knowledge of senior secondary school students in Nigeria about Head and Neck Cancer: Implications on prevention strategies. Malawi Med J. 2022;34(3):162-169. doi:10.4314/mmj.v34i3.4
- Oyapero A, Erinoso O, Olatosi O. A Community Survey on Association of Sociodemographic Characteristics with Risk Perception and Awareness about Oral Cancer in Lagos, Nigeria. Pesqui Bras Odontopediatria Clin Integr. 2024;24:1-12. doi:10.1590/pboci.2024.023
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
- Bernard W. Stewart and Christopher P. Wild, ed. World Cancer Report 2014. In: WHO. World Health Organization; 2015.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550-4559. doi:10.1200/JCO.2013.50.3870
- Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: Differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403. doi:10.1016/j.oraloncology.2014.01.016
- Arotiba JT, Obiechina AE, Fasola OA, Fawole OI, Ajagbe HA. Oral squamous cell carcinoma: a review of 246 Nigerian cases. Afr J Med Med Sci. 1999;28(3-4):141-144.
- Ladeinde AL, Adeyemo WL, Ogunlewe MO, Ajayi OF, Omitola OG. Salivary gland tumours: a 15-year review at the Dental Centre Lagos University Teaching Hospital. Afr J Med Med Sci. 2007;36(4):299-304.
- Brad Neville, Douglas D. Damm, Carl Allen, Angela Chi. Oral and Maxillofacial Pathology. In: Oral and Maxillofacial Pathology. 4th ed. Saunders; 2015. Accessed June 25, 2020. https://www.elsevier.com/books/oral-and-maxillofacial-pathology/neville/978-1-4557-7052-6
- Nwawolo CC, Ajekigbe AT, Oyeneyin JO, Nwankwo KC, Okeowo PA. Pattern of head and neck cancers among Nigerians in Lagos. West Afr J Med. 2001;20(2):111-116.
- Lawal AO, Kolude B, Adeyemi BF. Oral cancer: The Nigerian Experience. Int J Med Med Sci. 2013;5(4):178-183. doi:10.5897/IJMMS12.140
- Enwonwu CO, Meeks VI. Bionutrition and oral cancer in humans. Crit Rev oral Biol Med an Off Publ Am Assoc Oral Biol. 1995;6(1):5-17. doi:10.1177/10454411950060010401
- Okoh DS, Orikpete E, Omoregie OF, Ojo M. A STUDY OF THE CLINICOPATHOLOGIC PATTERNS OF OROFACIAL. Afr J Oral Maxillofac Path Med. 2015;1(2):9-16.
- Scully C. Rule for cancer diagnosis. Br Dent J. 2013;215(6):265-266. doi:10.1038/sj.bdj.2013.884
- Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8(9):11884-11894.
- Muller S, Tilakaratne WM. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022;16(1):54-62. doi:10.1007/s12105-021-01402-9
- Wood NK, Sawyer D, Goaz PW. Differential Diagnosis of Oral and Maxillofacial Lesions. Vol 53.; 2013.
- Winn DM, Lee YC, Hashibe M, et al. The INHANCE consortium: Toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Dis. 2015;21(6):685-693. doi:10.1111/odi.12342
- Gupta B, Johnson NW. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in south asia and the pacific. PLoS One. 2014;9(11). doi:10.1371/journal.pone.0113385
- La Vecchia C, Tavani A, Franceschi S, Levi F, Corrao G, Negri E. Epidemiology and prevention of oral cancer. Oral Oncol. 1997;33(5):302-312. doi:10.1016/S1368-8375(97)00029-8
- Macfarlane GJ, Zheng T, Marshall JR, et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer Part B Oral Oncol. 1995;31(3):181-187. doi:10.1016/0964-1955(95)00005-3
- Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J cancer. 2008;122(1):155-164. doi:10.1002/ijc.23033
- Lee YCA, Marron M, Benhamou S, et al. Active and involuntary tobacco smoking and upper aerodigestive tract cancer risks in a multicenter case-control study. Cancer Epidemiol biomarkers Prev a Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol. 2009;18(12):3353-3361. doi:10.1158/1055-9965.EPI-09-0910
- Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39(1):182-196. doi:10.1093/ije/dyp291
- Otoh EC, Johnson NW, Mandong BM, Danfillo IS. Primary head and neck cancers in Jos, Nigeria: a re-visit. West Afr J Med. 2006;25(2):92-100. doi:10.4314/wajm.v25i2.28256
- Odukoya O, Roberts T, Aroll G. A cytologic study of the effect of Kolanut on the keratinization of the palatal mucosal of Nigerian smokers. African Dent J Off Publ Fed African Dent Assoc = J Dent africain. 1990;4(1-5):1-5.
- Adebola RA, Bamgbose BO, Adeoye JB, Amole TG. Awareness of Oral Cancer in a Northwestern Nigerian State: Assessing the Knowledge, Opinion, and Practice of Traditional Healers and Herbalists. J Oral Oncol. 2013;2013:1-7. doi:10.1155/2013/263150